Inhibiting intracellular CD28 in cancer cells enhances antitumor immunity and overcomes anti-PD-1 resistance via targeting PD-L1
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Deciphering mechanisms for cancer immune escape may provide targets for improving immunotherapy efficacy. By in vivo genome-wide CRISPR loss-of-function screening in a mouse model of triple negative breast cancer (TNBC), we uncovered a non-classical function of Cd28 in cancer cells to promote immune escape. Knocking out Cd28 in cancer cells increased infiltration of type I conventional DC (cDC1) and activated tumor-specific CD8+ T cells, and pharmaceutical inducible knockdown of Cd28 inhibited pre-established tumor growth and overcame anti-PD-1 resistance in vivo. Furthermore, high expression of cancer cell CD28 in human TNBC tissues correlated with elevated PD-L1 expression, less CD8+ T cell infiltration, and poor prognosis. Mechanistically, intracellular CD28 directly bound to Cd274 mRNA and recruited spliceosomal factor SNRPB2 to stabilize Cd274 mRNA in nucleus, promoting PD-L1 expression and immune escape. Therefore, disrupting cancer cell CD28-mediated immune escape may provide a potential approach to improve breast cancer immunotherapy.
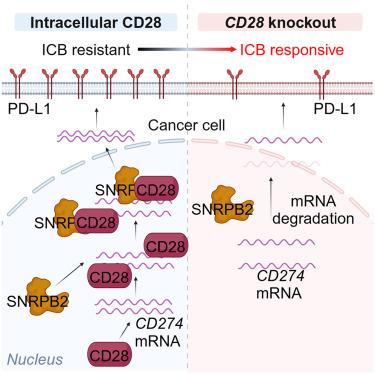
抑制癌细胞细胞内CD28增强抗肿瘤免疫,并通过靶向PD-L1克服抗pd -1耐药性
破解肿瘤免疫逃逸机制可能为提高免疫治疗效果提供靶点。通过在三阴性乳腺癌(TNBC)小鼠模型中进行体内全基因组CRISPR功能缺失筛查,我们发现Cd28在癌细胞中促进免疫逃逸的非经典功能。在癌细胞中敲除Cd28增加了I型常规DC (cDC1)的浸润并激活了肿瘤特异性CD8+ T细胞,药物诱导的Cd28敲除抑制了预先建立的肿瘤生长并克服了体内抗pd -1耐药性。此外,人类TNBC组织中癌细胞CD28的高表达与PD-L1表达升高、CD8+ T细胞浸润减少和预后不良相关。机制上,细胞内CD28直接结合Cd274 mRNA,募集剪接体因子SNRPB2稳定细胞核内Cd274 mRNA,促进PD-L1表达和免疫逃逸。因此,破坏癌细胞cd28介导的免疫逃逸可能提供一种改善乳腺癌免疫治疗的潜在途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
文献相关原料
公司名称
产品信息
索莱宝
G418
索莱宝
G418
Sigma
Polybrene
Sigma
DNase I
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


