First-Principles Investigation of Surface pKa and the Behavior of Acids at Aqueous–Metal Interfaces
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Several computational methods were reported for the accurate determination of pKa values in a solvent medium, but the research on surfaces and interfaces is limited. This study reports a new method for accurately determining the surface pKa (*pKa) of acids on surfaces. The *pKa is defined as the function of the adsorption energies of neutral acids and their deprotonated form. In the suggested method, the estimated proton solvation-free energy value is not required, a fact that increases the accuracy of the results. The *pKa values of various organic and inorganic acids on the (111) surfaces of Ag, Au, and Pt were evaluated. The results are validated with available experimental results on various surface coverage ratios. The results point out that weak acids adsorbed on the metal-aqueous interface are orders of magnitude stronger acids than those in homogeneous solutions. The shift of the pKas is largest on platinum. These results are of major importance in electrochemistry and heterogeneous catalysis.
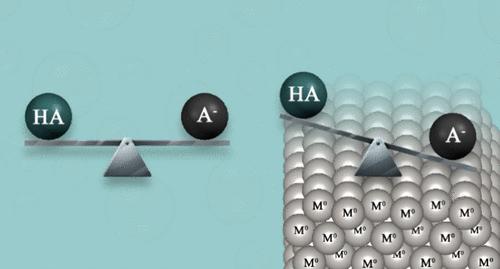
表面 pKa 和酸在水性金属界面行为的第一性原理研究
有几种计算方法可以精确测定溶剂介质中pKa值,但对表面和界面的研究有限。本研究报道了一种准确测定表面酸的pKa (*pKa)的新方法。*pKa定义为中性酸及其去质子化形式的吸附能的函数。在建议的方法中,不需要估计质子无溶剂化能值,这一事实提高了结果的准确性。测定了各种有机酸和无机酸在Ag、Au和Pt(111)表面的*pKa值。结果与已有的不同表面覆盖率的实验结果进行了验证。结果表明,吸附在金属-水界面上的弱酸要比吸附在均相溶液中的弱酸强几个数量级。铂金价格的变动幅度最大。这些结果在电化学和多相催化中具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


