Site-Specific Glyco-Tagging of Native Proteins for the Development of Biologicals
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
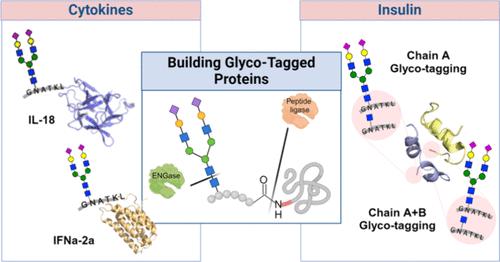
Glycosylation is an attractive approach to enhance biological properties of pharmaceutical proteins; however, the precise installation of glycans for structure–function studies remains challenging. Here, we describe a chemoenzymatic methodology for glyco-tagging of proteins by peptidoligase catalyzed modification of the N-terminus of a protein with a synthetic glycopeptide ester having an N-acetyl-glucosamine (GlcNAc) moiety to generate an N-GlcNAc modified protein. The GlcNAc moiety can be elaborated into complex glycans by trans-glycosylation using well-defined sugar oxazolines and mutant forms of endo β-N-acetylglucosaminidases (ENGases). The glyco-tagging methodology makes it possible to modify on-demand therapeutic proteins, including heterologous proteins expressed in E. coli, with diverse glycan structures. As a proof of principle, the N-terminus of interleukin (IL)-18 and interferon (IFN)α-2a was modified by a glycopeptide harboring a complex N-glycan without compromising biological potencies. The glyco-tagging methodology was also used to prepare several glycosylated insulin variants that exhibit reduced oligomerization, aggregation, and fibrillization yet maintained cell signaling properties, which are attractive for the development of insulins with improved shelf-lives. It was found that by employing different peptidoligases, it is possible to modify either the A or both chains of human insulin.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


