Spermine synthase engages in macrophages M2 polarization to sabotage antitumor immunity in hepatocellular carcinoma
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Disturbances in tumor cell metabolism reshape the tumor microenvironment (TME) and impair antitumor immunity, but the implicit mechanisms remain elusive. Here, we found that spermine synthase (SMS) was significantly upregulated in tumor cells, which correlated positively with the immunosuppressive microenvironment and predicted poor survival in hepatocellular carcinoma (HCC) patients. Via “subcutaneous” and “orthotopic” HCC syngeneic mouse models and a series of in vitro coculture experiments, we identified elevated SMS levels in HCC cells played a role in immune escape mainly through its metabolic product spermine, which induced M2 polarization of tumor-associated macrophages (TAMs) and subsequently corresponded with a decreased antitumor functionality of CD8+ T cells. Mechanistically, we discovered that spermine reprogrammed TAMs mainly by activating the PI3K-Akt-mTOR-S6K signaling pathway. Spermine inhibition in combination with immune checkpoint blockade effectively diminished tumor burden in vivo. Our results expand the understanding of the critical role of metabolites in regulating cancer progression and antitumor immunity and open new avenues for developing novel therapeutic strategies against HCC.

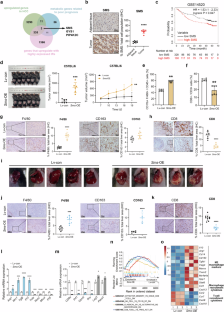
精胺合酶参与肝细胞癌巨噬细胞M2极化破坏抗肿瘤免疫
肿瘤细胞代谢紊乱重塑肿瘤微环境(TME)并损害抗肿瘤免疫,但其隐含机制尚不清楚。本研究中,我们发现精胺合酶(SMS)在肿瘤细胞中显著上调,与免疫抑制微环境呈正相关,并预测肝细胞癌(HCC)患者的生存率较低。通过“皮下”和“原位”HCC同基因小鼠模型以及一系列体外共培养实验,我们发现HCC细胞中SMS水平升高主要通过其代谢产物精胺在免疫逃逸中发挥作用,精胺诱导肿瘤相关巨噬细胞(tam)的M2极化,随后与CD8+ T细胞抗肿瘤功能下降相对应。在机制上,我们发现精胺主要通过激活PI3K-Akt-mTOR-S6K信号通路对tam进行重编程。精胺抑制联合免疫检查点阻断有效减轻体内肿瘤负荷。我们的研究结果扩大了对代谢物在调节癌症进展和抗肿瘤免疫中的关键作用的理解,并为开发针对HCC的新治疗策略开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


