Incidence of Richter transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma in the targeted therapy era
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0

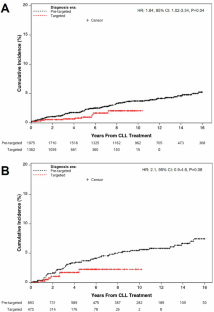
靶向治疗时代慢性淋巴细胞白血病/小淋巴细胞淋巴瘤Richter转化的发生率
Richter转化(RT)是慢性淋巴细胞白血病/小淋巴细胞淋巴瘤(CLL)向侵袭性淋巴瘤的组织学转化,最常见的是弥漫性大b细胞淋巴瘤(DLBCL)。RT对转化后的生存具有毁灭性的影响,通常为1年。RT频率的估计因研究设计、环境和随访时间而异。在使用化学免疫疗法(CIT)治疗CLL患者的几项大型研究中,在诊断后4-6年的中位随访后,RT率报道在2%至10%之间;这些研究既包括新诊断的CLL患者,也包括已治疗的CLL患者[2,3,4,5,6]。靶向治疗,包括布鲁顿酪氨酸激酶抑制剂(BTKi)和b细胞淋巴瘤2抑制剂(BCL2i),现在是CLL患者的标准护理治疗。BTKi和BCL2i在复发CLL患者中的早期研究中报道的高RT率(高达25%)可能反映了大量预处理患者RT风险的增加,而不是由于这些治疗本身的风险[7,8]。CIT和靶向治疗组一线临床试验特异性治疗暴露后的风险比较受到报道的低RT事件数量的限制[9,10,11,12,13]。由于CLL的治疗模式发生了变化,CLL患者的寿命更长,我们旨在通过比较不同时期新诊断的CLL患者队列,评估目前发展为RT的风险以及靶向治疗对该风险的潜在影响。在IRB批准后,我们在梅奥诊所CLL数据库中发现了诊断后12个月内未治疗的CLL患者。只有在梅奥诊所活检证实DLBCL并经组织病理学证实的病例才被认为是RT事件。累积发病率方法学用于显示从初始CLL诊断时间和从CLL定向治疗开始到RT发展的时间,死亡是一个竞争风险。我们将2014年2月(FDA批准ibrutinib治疗CLL)之前的时期定义为预靶向治疗时代,2014年2月之后的时期定义为靶向治疗时代。使用Cox比例风险回归分析,我们比较了靶向治疗前和靶向治疗时期诊断为CLL患者的RT发生率。还采用Cox回归分析来调查治疗暴露类型(时间相关变量)对rt风险的影响。使用SAS 9.4 (SAS Institute, Cary, NC, USA)进行统计分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


