Amide-Based Naphthotubes as Biomimetic Receptors for Acetal Protection and Other Substrates in Water via Noncovalent Interactions
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
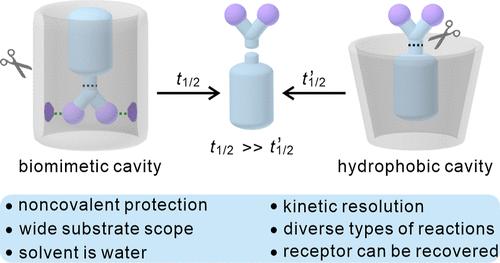
Active compound protection can allow inherently unstable molecules to be stabilized and latent reactivity to be masked. Synthetic receptors are attractive in terms of providing such protection. Nevertheless, preserving the activity and functionality of organic molecules in water poses a challenge. Here, we show that biomimetic receptors, specifically amide naphthotubes and an amide anthryltube, allow the efficient preservation of functional organic molecules in water. In particular, the amide naphthotubes were found to extend the half-lives of acetal-containing substrates (“acetals”) against acid-catalyzed hydrolysis by up to 3000 times. This kinetic protection effect was ascribed to hydrogen bond-based recognition of the organic guests. A substrate dependence was seen that was further exploited to achieve the kinetic resolution of acetal isomers. To the best of our knowledge, the present study constitutes one of the most effective acetal protection strategies reported to date. The recognition-based protection approach reported here appears generalizable as evidenced by the protection of eight different substrates against six distinct chemical reactions. Based on the present findings, we propose that it is possible to design receptors that provide for the protection of specific substrates under a variety of reaction conditions including those carried out in water.
酰胺基萘管通过非共价相互作用作为水中缩醛保护和其他底物的仿生受体
活性化合物保护可以使固有不稳定的分子得到稳定,并掩盖潜在的反应性。合成受体在提供这种保护方面是有吸引力的。然而,保持水中有机分子的活性和功能是一个挑战。在这里,我们证明了仿生受体,特别是酰胺萘管和酰胺蒽管,可以有效地保存水中的功能有机分子。特别是,酰胺萘管被发现可以延长含缩醛底物(“缩醛”)的半衰期,使其抗酸催化水解的半衰期延长3000倍。这种动力学保护作用归因于对有机客体的氢键基识别。对底物的依赖性被进一步利用来实现缩醛异构体的动力学分辨。据我们所知,目前的研究是迄今为止报道的最有效的缩醛保护策略之一。这里报道的基于识别的保护方法似乎是可推广的,八种不同的底物对六种不同的化学反应的保护证明了这一点。基于目前的研究结果,我们提出有可能设计出在各种反应条件下(包括在水中进行的反应)提供特定底物保护的受体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


