Asymmetric N-Glycosylation in the Tailpiece of Recombinant IgA1
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
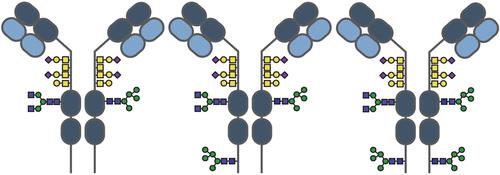
Here, we employed a variety of mass spectrometry (MS)-based approaches, both (glyco)peptide-centric and protein-centric, to resolve the complex glycoproteoform landscape of recombinant IgA1 produced in HEK293 cells. These key immunoglobulins harbor several N- and O-glycosylation sites, making them considerably more heterogeneous than their IgG counterparts. We provide quantitative data on the occupancy and glycan composition for each IgA1 glycosylation site. Combining all data, we revealed that IgA1 molecules consist of at least three distinct populations with varying N-glycosylation site occupancies at the C-terminal tailpiece, namely, one with both glycosylation sites occupied, another with both glycosylation sites unoccupied, and a third asymmetric population with one glycosylation site occupied and the other unoccupied, challenging the prevailing acceptance that IgA1 N-glycosylation is symmetrical. This finding is significant, given that the tailpiece is involved in interactions with the J-chain and the Polymeric Immunoglobulin Receptor, and in general as antibody glycosylation is a quality attribute that needs to be carefully monitored, as the presence and nature of these modifications can affect the antibody’s efficacy, lifetime, stability, and binding and/or neutralizing capacities. Optimizing strategies to produce recombinant IgA1 requires efficient and specific quality control analytical strategies, as presented here, which is essential for therapeutic IgA1-based antibody development. We expect that the integrated MS-based strategy presented here may be beneficial to comprehensively characterize the glycoproteoform profiles of IgA1-based therapeutics, thereby improving their production and optimization processes and facilitating the pathway to bring more IgA1-based therapeutics into clinical applications.
重组IgA1尾部的不对称n -糖基化
在这里,我们采用了多种质谱(MS)为基础的方法,以(糖)肽为中心和以蛋白为中心,来解析HEK293细胞中产生的重组IgA1的复杂糖蛋白形态。这些关键的免疫球蛋白含有几个N-和o -糖基化位点,使它们比它们的IgG对应物更具异质性。我们提供了每个IgA1糖基化位点的占用和聚糖组成的定量数据。结合所有数据,我们发现IgA1分子由至少三个不同的群体组成,这些群体在c端尾部的n -糖基化位点占据不同,即两个糖基化位点都被占据,另一个糖基化位点都没有被占据,第三个不对称群体一个糖基化位点被占据,另一个没有被占据,挑战了普遍接受的IgA1 n -糖基化是对称的。这一发现意义重大,因为尾部与j链和聚合免疫球蛋白受体的相互作用有关,一般来说,抗体糖基化是一个需要仔细监测的质量属性,因为这些修饰的存在和性质会影响抗体的功效、寿命、稳定性、结合和/或中和能力。优化生产重组IgA1的策略需要高效和特定的质量控制分析策略,正如本文所述,这对于基于IgA1的治疗性抗体开发至关重要。我们期望本文提出的基于ms的综合策略可能有助于全面表征基于iga1的治疗药物的糖蛋白形态特征,从而改善其生产和优化过程,并促进更多基于iga1的治疗药物进入临床应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


