Mechanically mixing copper and iron at subnanometric scale for catalyzing hydrogen evolution reaction
IF 9.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Economically viable catalysts with high activity and stability are highly demanded for water electrolysis. Herein, we choose low-cost copper and iron as the raw materials, and mechanically mix them into at the subnanometric scale to obtain a self-supporting electrode. The interaction of copper and iron optimizes the d-band center and adsorptive capability of the catalyst, thus significantly improving the catalytic properties of hydrogen evolution reaction (HER). The CuFe catalyst achieves ultralow overpotentials of 67 mV at 10 mA cm−2 and 687 mV at 1000 mA cm−2. As well, the catalyst presents excellent stability, with only a 3.03 % drop in the initial current density after 1000 h of operation at 1 A cm−2. Compared to nickel-based catalysts, the CuFe catalyst proves to be superior in terms of catalytic activity, stability, and price, showing great potential on industrial applications.
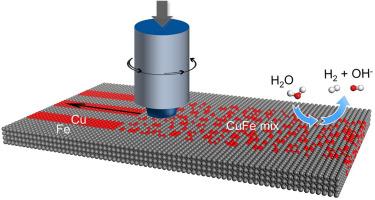

在亚纳米尺度上机械混合铜和铁,催化析氢反应
电解水需要经济可行、活性高、稳定性好的催化剂。本文选择低成本的铜和铁作为原料,在亚纳米尺度上进行机械混合,得到一种自支撑电极。铜和铁的相互作用优化了催化剂的d带中心和吸附能力,从而显著提高了析氢反应(HER)的催化性能。CuFe催化剂在10 mA cm - 2和1000 mA cm - 2下的过电位分别为67 mV和687mv。此外,催化剂表现出优异的稳定性,在1 a cm−2下工作1000小时后,初始电流密度仅下降3.03%。与镍基催化剂相比,CuFe催化剂在催化活性、稳定性和价格上都具有优势,具有很大的工业应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Materialia
工程技术-材料科学:综合
CiteScore
16.10
自引率
8.50%
发文量
801
审稿时长
53 days
期刊介绍:
Acta Materialia serves as a platform for publishing full-length, original papers and commissioned overviews that contribute to a profound understanding of the correlation between the processing, structure, and properties of inorganic materials. The journal seeks papers with high impact potential or those that significantly propel the field forward. The scope includes the atomic and molecular arrangements, chemical and electronic structures, and microstructure of materials, focusing on their mechanical or functional behavior across all length scales, including nanostructures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


