Programmable Circular Multispecific Aptamer-Drug Engager to Broadly Boost Antitumor Immunity
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Safely and effectively harnessing innate immunity to boost cancer immunotherapy is promising yet challenging. Hence, we have developed a series of programmable aptamer-based multispecific engagers by encoding various artificial aptamer-drug codons with DNA-templated polymerization, aiming to broadly boost innate and adaptive immunity for antitumor therapy. All circular single-stranded multivalent aptamer-drug conjugates (os-mvApDCs) had a dendritic structure, precise size, and excellent stability, enabling prolonged blood circulation, targeted tumor accumulation, and rapid multireceptor-mediated endocytosis. A trispecific engager (Sl/Pd/Mjos-mvApDCsSMT), targeting PD-1 on CD8+ T cells and PD-L1/c-Met on tumor cells, recruited large amounts of immune cells into the tumor and released cytotoxic MMAE and immunomodulators, inducing severe cell death and broad activation of innate immunity. When combined with the αPD-1 blockade, there was a significant increase in the number of CD8+ T cells (10-fold increase versus untreated control) engaged and expanded in the tumor, exhibiting potent function (IFN-γ+/GzmB+) and low exhaustion (PD-1+TIM-3+). The orchestrated innate and adaptive immunity effectively eliminated immunosuppressive MDSCs, Tregs, and M2-like macrophages in tumors and promoted the maturation of dendritic cells (DCs) in the draining lymph nodes, resulting in robust and durable systemic antitumor efficacy, with 7 out of 8 mice surviving over 60 days. Our programmable DNA-templated printing technology enables the rational design of multispecific therapeutics with modular composition and function but minimal production issues, providing a versatile tool for the development of multifunctional personalized medicine.
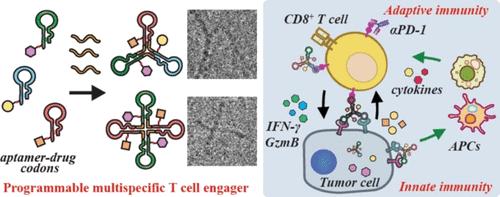
可编程圆形多特异性适配体-药物接合器广泛增强抗肿瘤免疫
安全有效地利用先天免疫来促进癌症免疫治疗是有希望的,但也具有挑战性。因此,我们开发了一系列可编程的基于适配体的多特异性接合体,通过dna模板聚合编码各种人工适配体药物密码子,旨在广泛提高抗肿瘤治疗的先天和适应性免疫。所有环状单链多价适配体-药物偶联物(os-mvApDCs)都具有树突状结构、精确的尺寸和出色的稳定性,能够延长血液循环、靶向肿瘤积累和快速多受体介导的内吞作用。一种三特异性接合子(Sl/Pd/ mjo - mvapdcssmt),靶向CD8+ T细胞上的Pd -1和肿瘤细胞上的Pd - l1 /c-Met,招募大量免疫细胞进入肿瘤并释放细胞毒性MMAE和免疫调节剂,诱导严重的细胞死亡和先天免疫的广泛激活。当与αPD-1阻断剂联合使用时,CD8+ T细胞在肿瘤中参与和扩增的数量显著增加(比未治疗对照组增加10倍),表现出强大的功能(IFN-γ+/GzmB+)和低衰竭(PD-1+TIM-3+)。精心安排的先天免疫和适应性免疫有效地消除了肿瘤中免疫抑制的MDSCs、Tregs和m2样巨噬细胞,促进了引流淋巴结中树突状细胞(DCs)的成熟,从而产生了强大而持久的全身抗肿瘤功效,8只小鼠中有7只存活超过60天。我们的可编程dna模板打印技术能够合理设计具有模块化组成和功能的多特异性治疗药物,但生产问题最少,为多功能个性化医疗的发展提供了一个多功能工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


