Mannose metabolism reshapes T cell differentiation to enhance anti-tumor immunity
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Cellular metabolic status profoundly influences T cell differentiation, persistence, and anti-tumor efficacy. Our single-cell metabolic analyses of T cells reveal that diminished mannose metabolism is a prominent feature of T cell dysfunction. Conversely, experimental augmentation/restoration of mannose metabolism in adoptively transferred T cells via D-mannose supplementation enhances anti-tumor activity and restricts exhaustion differentiation both in vitro and in vivo. Mechanistically, D-mannose treatment induces intracellular metabolic programming and increases the O-GlcNAc transferase (OGT)-mediated O-GlcNAcylation of β-catenin, which preserves Tcf7 expression and epigenetic stemness, thereby promoting stem-like programs in T cells. Furthermore, in vitro expansion with D-mannose supplementation yields T cell products for adoptive therapy with stemness characteristics, even after extensive long-term expansion, that exhibits enhanced anti-tumor efficacy. These findings reveal cell-intrinsic mannose metabolism as a physiological regulator of CD8+ T cell fate, decoupling proliferation/expansion from differentiation, and underscoring the therapeutic potential of mannose modulation in cancer immunotherapy.
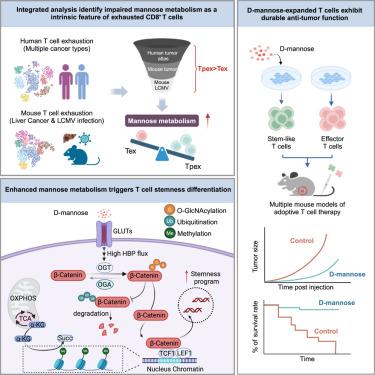
甘露糖代谢重塑T细胞分化,增强抗肿瘤免疫
细胞代谢状态深刻影响T细胞的分化、持久性和抗肿瘤功效。我们对T细胞的单细胞代谢分析表明,甘露糖代谢减少是T细胞功能障碍的一个突出特征。相反,在实验中,通过补充d -甘露糖来增强/恢复过继转移T细胞的甘露糖代谢,可以增强抗肿瘤活性,并限制体外和体内的衰竭分化。从机制上说,d -甘露糖处理诱导细胞内代谢编程,增加O-GlcNAc转移酶(OGT)介导的β-catenin的o - glcn酰化,从而保持Tcf7的表达和表观遗传干性,从而促进T细胞中的干样程序。此外,补充d -甘露糖的体外扩增产生具有干细胞特性的过继治疗T细胞产品,即使经过广泛的长期扩增,也显示出增强的抗肿瘤功效。这些发现揭示了细胞内在甘露糖代谢作为CD8+ T细胞命运的生理调节剂,将增殖/扩增与分化解耦,并强调了甘露糖调节在癌症免疫治疗中的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


