EZH2 inhibition enhances T cell immunotherapies by inducing lymphoma immunogenicity and improving T cell function
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
T cell-based immunotherapies have demonstrated effectiveness in treating diffuse large B cell lymphoma (DLBCL) and follicular lymphoma (FL) but predicting response and understanding resistance remains a challenge. To address this, we developed syngeneic models reflecting the genetics, epigenetics, and immunology of human FL and DLBCL. We show that EZH2 inhibitors reprogram these models to re-express T cell engagement genes and render them highly immunogenic. EZH2 inhibitors do not harm tumor-controlling T cells or CAR-T cells. Instead, they reduce regulatory T cells, promote memory chimeric antigen receptor (CAR) CD8 phenotypes, and reduce exhaustion, resulting in a decreased tumor burden. Intravital 2-photon imaging shows increased CAR-T recruitment and interaction within the tumor microenvironment, improving lymphoma cell killing. Therefore, EZH2 inhibition enhances CAR-T cell efficacy through direct effects on CAR-T cells, in addition to rendering lymphoma B cells immunogenic. This approach is currently being evaluated in two clinical trials, NCT05934838 and NCT05994235, to improve immunotherapy outcomes in B cell lymphoma patients.
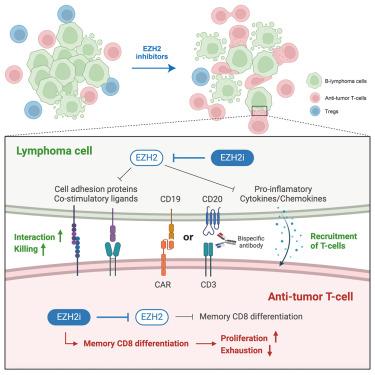
EZH2抑制通过诱导淋巴瘤免疫原性和改善T细胞功能来增强T细胞免疫治疗
基于T细胞的免疫疗法已经证明在治疗弥漫性大B细胞淋巴瘤(DLBCL)和滤泡性淋巴瘤(FL)方面是有效的,但预测反应和了解耐药性仍然是一个挑战。为了解决这个问题,我们建立了反映人类FL和DLBCL的遗传学、表观遗传学和免疫学的同基因模型。我们发现EZH2抑制剂对这些模型进行了重编程,以重新表达T细胞接合基因,并使它们具有高度的免疫原性。EZH2抑制剂不会损害肿瘤控制T细胞或CAR-T细胞。相反,它们减少调节性T细胞,促进记忆嵌合抗原受体(CAR) CD8表型,减少衰竭,从而减少肿瘤负担。活体双光子成像显示肿瘤微环境中CAR-T募集和相互作用增加,改善淋巴瘤细胞杀伤。因此,EZH2抑制除了使淋巴瘤B细胞具有免疫原性外,还通过直接作用于CAR-T细胞来增强CAR-T细胞的功效。该方法目前正在两项临床试验NCT05934838和NCT05994235中进行评估,以改善B细胞淋巴瘤患者的免疫治疗结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


