Exploring the Impact of Carbazole Position on Thermally Activated Delayed Fluorescence and Room-Temperature Phosphorescence Properties in Phthalimide–Carbazole Conjugates: A Density-Functional Theory Study
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aromatic imide-based thermally activated delayed fluorescence (TADF) emitters have become increasingly popular due to their unique properties such as rigid structures, pronounced thermal stability, strong electron-withdrawing ability, and exceptional photoluminescence characteristics. In this work, the phthalimide unit is integrated with the carbazole donor in different molecular designs (D-π-A, π-A-D, π-A-D2, and D-π-A-D) and their TADF and room-temperature phosphorescence (RTP) properties are theoretically investigated. Descriptors such as the energy gap between singlet and triplet excited states (ΔEST), spin orbit coupling (SOC), charge transfer (CT) indices, and rate constants of excited state processes were analyzed to study the nature of the excited state. The D-π-A and D-π-A-D molecules possess small ΔEST, CT-dominated S1 and T1 states, relatively low SOC values, and high radiative and reverse intersystem crossing (RISC) rates, while the π-A-D and π-A-D2 molecules with direct substitution of carbazole on phthalimide core showed large ΔEST, CT-dominated S1 state, hybridized and local charge transfer T1 state, large SOC, high intersystem crossing, and low RISC rates. Careful analysis of energy-level diagram and hole–electron distribution revealed the role of the closely lying T2 state with the S1 state in fast up-conversion of triplet excitons through a multichannel RISC process in π-A-D2- and D-π-A-D-based molecules. Overall, the D-π-A and D-π-A-D molecules possess parameters indicative of TADF emission, and the π-A-D and π-A-D2 molecules inherit parameters facilitating RTP emission. Additionally, the π-A-D2 molecules can display TADF emission; on the contrary, the π-A-D molecules display poor TADF propensity.
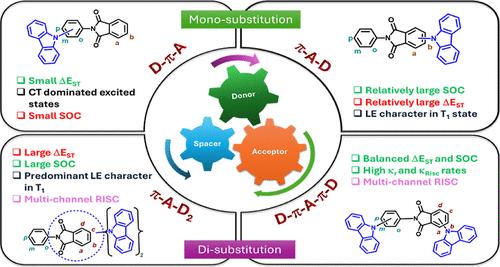
探讨咔唑位置对邻苯二胺-咔唑偶联物热激活延迟荧光和室温磷光性质的影响:密度泛函理论研究
芳香族亚胺基热激活延迟荧光(TADF)发射器由于其独特的性质,如刚性结构,明显的热稳定性,强的吸电子能力,以及优异的光致发光特性而越来越受欢迎。本文将邻苯二甲酸亚胺单元与咔唑供体以不同的分子设计(D-π-A, π-A-D, π-A-D2, D-π-A-D)相结合,从理论上研究了它们的TADF和室温磷光(RTP)性能。分析了单线态和三重态激发态之间的能隙(ΔEST)、自旋轨道耦合(SOC)、电荷转移(CT)指数和激发态过程的速率常数等描述符,研究了激发态的性质。D-π-A和D-π-A-D分子具有较小的ΔEST、ct主导的S1态和T1态,SOC值相对较低,辐射和反向系统间交叉(RISC)速率高;而邻苯二胺核上直接取代咔唑的π-A-D和π-A-D2分子具有较大的ΔEST、ct主导的S1态,杂化和局部电荷转移T1态,SOC大,系统间交叉高,RISC速率低。通过对能级图和空穴电子分布的仔细分析,揭示了在π-A-D2和D-π- a -D基分子中,紧密相连的T2态和S1态在三重态激子通过多通道RISC过程快速上转换中的作用。总的来说,D-π-A和D-π-A-D分子具有指示TADF发射的参数,而π-A-D和π-A-D2分子继承了有利于RTP发射的参数。此外,π-A-D2分子可以显示TADF发射;相反,π-A-D分子表现出较差的TADF倾向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


