A Demethylation-Switchable Aptamer Design Enables Lag-Free Monitoring of m6A Demethylase FTO with Energy Self-Sufficient and Structurally Integrated Features
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cellular context profiling of modification effector proteins is critical for an in-depth understanding of their biological roles in RNA N6-methyladenosine (m6A) modification regulation and function. However, challenges still remain due to the high context complexities, which call for a versatile toolbox for accurate live-cell monitoring of effectors. Here, we propose a demethylation-switchable aptamer sensor engineered with a site-specific m6A (DSA-m6A) for lag-free monitoring of the m6A demethylase FTO activity in living cells. As a proof of concept, a DNA aptamer against adenosine triphosphate (ATP) is selected to construct the DSA-m6A model, as the “universal energy currency” role of ATP could guarantee the equally fast and spontaneous conformation change of DSA-m6A sensor upon demethylation and ATP binding in living organisms, thus enabling sensitive monitoring of FTO activity with neither time delay nor recourse to extra supply of substances. This ATP-driven DSA-m6A design facilitates biomedical research, including live-cell imaging, inhibitor screening, single-cell tracking of dynamic FTO nuclear translocation upon starvation stimuli, FTO characterization in a biomimetic heterotypic three-dimensional (3D) multicellular spheroid model, as well as the first report on the in vivo imaging of FTO activity. This strategy provides a simple yet versatile toolbox for clinical diagnosis, drug discovery, therapeutic evaluation, and biological study of RNA demethylation.
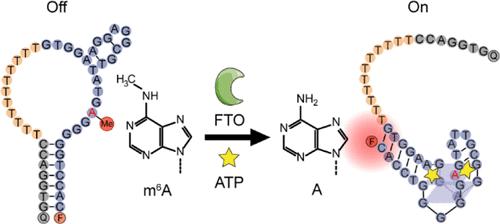
具有能量自给自足和结构集成功能的去甲基化适配体设计使m6A去甲基化酶FTO的无滞后监测成为可能
修饰效应蛋白的细胞背景分析对于深入了解其在RNA n6 -甲基腺苷(m6A)修饰调控和功能中的生物学作用至关重要。然而,由于环境的高度复杂性,挑战仍然存在,这需要一个多功能工具箱来精确监测效应器的活细胞。在这里,我们提出了一种带有位点特异性m6A (DSA-m6A)的去甲基化可切换适配体传感器,用于无滞后监测活细胞中m6A去甲基化酶FTO活性。为了验证这一概念,我们选择了一个抗三磷酸腺苷(adenosine triphosphate, ATP)的DNA适体来构建DSA-m6A模型,因为ATP的“通用能量货币”作用可以保证DSA-m6A传感器在生物体中去甲基化和ATP结合时同样快速和自发的构象变化,从而实现对FTO活性的敏感监测,不需要时间延迟,也不需要额外的物质供应。这种atp驱动的DSA-m6A设计促进了生物医学研究,包括活细胞成像、抑制剂筛选、饥饿刺激下动态FTO核易位的单细胞跟踪、FTO在仿生异型三维(3D)多细胞球体模型中的表征,以及FTO活性的体内成像的第一份报告。该策略为临床诊断、药物发现、治疗评估和RNA去甲基化的生物学研究提供了一个简单而通用的工具箱。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


