Synthetic Study toward Daphnimacropodines
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Daphnimacropodines A-C are members of a small but structurally distinct subfamily of Daphniphyllum alkaloids. Their congested polycyclic skeletons, and two vicinal quaternary stereocenters, present significant synthetic challenges. This paper describes two stereoselective approaches to constructing the tricyclic core structures of daphnimacropodines, achieved through a straightforward Rh-catalyzed [4 + 3] cycloaddition using simple building blocks. This work also highlights an intramolecular Heck reaction that rapidly assembles the cyclohexane ring moiety, a Tsuji-Trost allylation that forged the critical C-8 quaternary stereocenter, an efficient hetero-Diels–Alder reaction, and an intramolecular nucleophilic addition, which paved the way to the key cyclopentane ring. The assembly of the tetrahydropyrrole motif was also investigated.
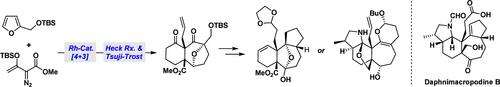
水蚤丙足碱的合成研究
Daphnimacropodines a - c是水蚤属生物碱的一个小而结构独特的亚家族的成员。他们拥挤的多环骨架,和两个相邻的四元立体中心,提出了重大的合成挑战。本文描述了两种立体选择的方法来构建三环核心结构的daphnimacropodine,通过一个直接的铑催化[4 + 3]环加成实现使用简单的构建块。这项工作还强调了快速组装环己烷环部分的分子内Heck反应,形成关键C-8季立体中心的Tsuji-Trost烯丙化反应,高效的杂diols - alder反应,以及为关键环戊烷环铺平道路的分子内亲核加成。对四氢吡咯基序的组装也进行了研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


