p53 induces circFRMD4A to suppress cancer development through glycolytic reprogramming and cuproptosis
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
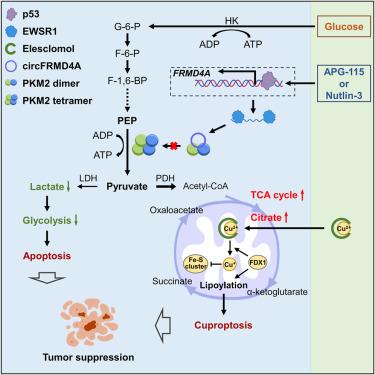
Cuproptosis is a type of copper-induced cell death that mainly impacts cells relying on mitochondrial metabolism. Although p53 regulates glycolytic metabolism, its role in cuproptosis remains unclear. Here, we report that the circular RNA, circFRMD4A, is crucial for p53-mediated metabolic reprogramming and cuproptosis. CircFRMD4A originates from the transcript of FRMD4A, which is transcriptionally activated by p53, and the formation of circFRMD4A is facilitated by the RNA-binding protein EWSR1. CircFRMD4A functions as a tumor suppressor and enhances the sensitivity of cancer cells to elesclomol-induced cuproptosis. Mechanistic analysis reveals that circFRMD4A interacts with and inactivates the pyruvate kinase PKM2, leading to a decrease in lactate production and a redirection of glycolytic flux toward the tricarboxylic acid cycle. Finally, p53 agonists and elesclomol coordinately suppress the growth of cancer in a xenograft mouse model. Altogether, our study uncovers that p53 promotes glycolytic reprogramming and cuproptosis via circFRMD4A and suggests a potential combination strategy against cancers with wild-type p53.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


