Leveraging natural language processing to aggregate field safety notices of medical devices across the EU
IF 12.4
1区 医学
Q1 HEALTH CARE SCIENCES & SERVICES
引用次数: 0
Abstract
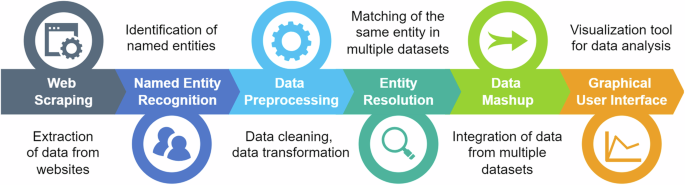
The European Union (EU) Medical Device Regulation and In Vitro Medical Device Regulation have introduced more rigorous regulatory requirements for medical devices, including new rules for post-market surveillance. However, EU market vigilance is limited by the absence of harmonized reporting systems, languages and nomenclatures among Member States. Our aim was to develop a framework based on Natural Language Processing capable of automatically collecting publicly available Field Safety Notices (FSNs) reporting medical device problems by applying web scraping to EU authority websites, to attribute the most suitable device category based on the European Medical Device Nomenclature (EMDN), and to display processed FSNs in an aggregated way to allow multiple queries. 65,036 FSNs published up to 31/12/2023 were retrieved from 16 EU countries, of which 40,212 (61.83%) were successfully assigned the proper EMDN. The framework’s performance was successfully tested, with accuracies ranging from 87.34% to 98.71% for EMDN level 1 and from 64.15% to 85.71% even for level 4.

求助全文
约1分钟内获得全文
求助全文
来源期刊

NPJ Digital Medicine
Multiple-
CiteScore
25.10
自引率
3.30%
发文量
170
审稿时长
15 weeks
期刊介绍:
npj Digital Medicine is an online open-access journal that focuses on publishing peer-reviewed research in the field of digital medicine. The journal covers various aspects of digital medicine, including the application and implementation of digital and mobile technologies in clinical settings, virtual healthcare, and the use of artificial intelligence and informatics.
The primary goal of the journal is to support innovation and the advancement of healthcare through the integration of new digital and mobile technologies. When determining if a manuscript is suitable for publication, the journal considers four important criteria: novelty, clinical relevance, scientific rigor, and digital innovation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


