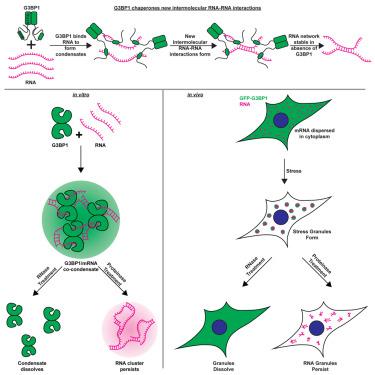
G3BP1 promotes intermolecular RNA-RNA interactions during RNA condensation
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Ribonucleoprotein (RNP) granules are biomolecular condensates requiring RNA and proteins to assemble. Stress granules are RNP granules formed upon increases in non-translating messenger ribonucleoprotein particles (mRNPs) during stress. G3BP1 and G3BP2 proteins are proposed to assemble stress granules through multivalent crosslinking of RNPs. We demonstrate that G3BP1 also has “condensate chaperone” functions, which promote the assembly of stress granules but are dispensable following initial condensation. Following granule formation, G3BP1 is dispensable for the RNA component of granules to persist in vitro and in cells when RNA decondensers are inactivated. These results demonstrate that G3BP1 functions as an “RNA condenser,” a protein that promotes intermolecular RNA-RNA interactions stabilizing RNA condensates, leading to RNP granule persistence. Moreover, the stability of RNA-only granules highlights the need for active mechanisms limiting RNP condensate stability and lifetime.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


