HMX3 is a critical vulnerability in MECOM-negative KMT2A::MLLT3 acute myelomonocytic leukemia
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
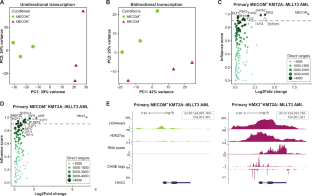
KMT2A::MLLT3 acute myelomonocytic leukemia (AML) comes in two clinically and biologically different subtypes. One is characterized by inferior outcome, older age, and MECOM oncogene expression. The other is mainly observed in children and young adults, associates with better clinical outcome, but lacks MECOM. To identify cell fate determining transcription factors downstream of KMT2A::MLLT3, we applied a bioinformatic algorithm that integrates gene and enhancer expression from primary MECOM-positive and -negative KMT2A::MLLT3 AML samples. This identified MECOM to be most influential in the MECOM-positive group, while neuronal transcription factor HMX3 was most influential in the MECOM-negative group. In large AML cohorts, HMX3 expression associated with a unique gene expression profile, younger age (p < 0.002) and KMT2A-rearranged and KAT6A-CREBBP leukemia (p < 0.00001). HMX3 was not expressed in other major genetic risk groups and healthy blood cells. RNA-sequencing analyses following forced HMX3 expression in healthy CD34+ cells and its silencing in KMT2A::MLT3 cells showed that HMX3 drives cancer-associated E2F and MYC gene programs (p < 0.001). HMX3 expression in healthy CD34+ cells blocked monocytic but not granulocytic colony formation. Strikingly, HMX3 silencing in KMT2A::MLLT3 patient cells resulted in cell cycle arrest, monocytic differentiation and apoptosis. Thus, the neuronal transcription factor HMX3 is a leukemia-specific vulnerability in KMT2A::MLLT3 AML.

HMX3是mecom阴性KMT2A::MLLT3急性髓单细胞白血病的一个关键易感性
KMT2A::MLLT3急性髓细胞白血病(AML)有两种临床和生物学上不同的亚型。一种以预后差、年龄大、MECOM癌基因表达为特征。另一种主要在儿童和年轻人中观察到,与较好的临床结果相关,但缺乏MECOM。为了鉴定KMT2A::MLLT3下游决定细胞命运的转录因子,我们应用了一种生物信息学算法,整合了mecom阳性和阴性KMT2A::MLLT3 AML样品的基因和增强子表达。这表明MECOM在MECOM阳性组中影响最大,而神经元转录因子HMX3在MECOM阴性组中影响最大。在大型AML队列中,HMX3表达与独特的基因表达谱、年轻(p < 0.002)和kmt2a -重排和KAT6A-CREBBP白血病(p < 0.00001)相关。HMX3在其他主要遗传风险组和健康血细胞中不表达。在健康CD34+细胞中强制表达HMX3及其在KMT2A::MLT3细胞中的沉默后的rna测序分析显示,HMX3驱动癌症相关的E2F和MYC基因程序(p < 0.001)。HMX3在健康CD34+细胞中的表达阻断单核细胞集落形成,但不阻断粒细胞集落形成。引人注目的是,在KMT2A::MLLT3患者细胞中,HMX3沉默导致细胞周期阻滞、单核细胞分化和凋亡。因此,神经元转录因子HMX3在KMT2A::MLLT3 AML中是白血病特异性易感性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


