A Probe Molecule Based on 1,3,4-Oxadiazole Constructed for Crystal Structure Analysis and Sn4+ Identification
IF 1.4
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
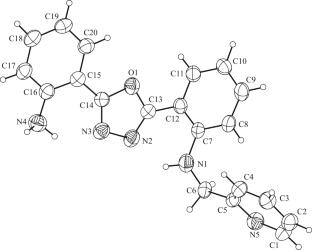
In order to develop the application of 1,3,4-oxadiazole compounds in the direction of fluorescent probes, we modified the probes by introducing 2-(bromomethyl)pyridine on the amino group of 1,3,4-oxadiazole derivatives, and synthesized HL1(2-(5-(2-aminophenyl)-1,3,4-oxadiazol-2-yl)-N-(pyridin-2-ylmethyl)aniline). Its structure was characterized by NMR, HRMS and single crystal X-ray diffraction. The weak interaction of HL1 was analyzed by using Multiwfn to draw Hirshfeld surface. The HOMO and LUMO orbitals of HL1 were analyzed in B3LYP/6-311++G basis set using DFT theory. HL1 is highly selective to Sn4+ in ACN, LOD = 5.08∙10–7 M, Ka = 1.73∙103M–1.
基于1,3,4-恶二唑的探针分子用于晶体结构分析和Sn4+鉴定
为了开发1,3,4-恶二唑类化合物在荧光探针方面的应用,我们在1,3,4-恶二唑衍生物的氨基上引入2-(溴甲基)吡啶对探针进行修饰,合成了HL1(2-(5-(2-氨基苯基)-1,3,4-恶二唑-2-基)- n-(吡啶-2-基甲基)苯胺)。通过NMR、HRMS和单晶x射线衍射对其结构进行了表征。利用Multiwfn绘制Hirshfeld曲面,分析了HL1的弱相互作用。利用DFT理论,在B3LYP/6-311++G基集中分析了HL1的HOMO和LUMO轨道。HL1在ACN中对Sn4+具有高度选择性,LOD = 5.08∙10-7 M, Ka = 1.73∙103M-1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Structural Chemistry
化学-无机化学与核化学
CiteScore
1.60
自引率
12.50%
发文量
142
审稿时长
8.3 months
期刊介绍:
Journal is an interdisciplinary publication covering all aspects of structural chemistry, including the theory of molecular structure and chemical bond; the use of physical methods to study the electronic and spatial structure of chemical species; structural features of liquids, solutions, surfaces, supramolecular systems, nano- and solid materials; and the crystal structure of solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


