Molecular connectomics reveals a glucagon-like peptide 1-sensitive neural circuit for satiety
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
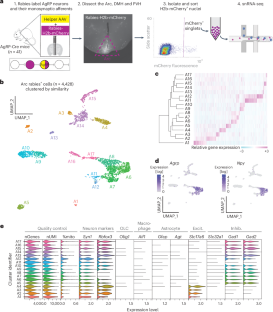
Liraglutide and other glucagon-like peptide 1 receptor agonists (GLP-1RAs) are effective weight loss drugs, but how they suppress appetite remains unclear. One potential mechanism is by activating neurons that inhibit the hunger-promoting Agouti-related peptide (AgRP) neurons of the arcuate hypothalamus (Arc). To identify these afferents, we developed a method combining rabies-based connectomics with single-nucleus transcriptomics. Here, we identify at least 21 afferent subtypes of AgRP neurons in the mouse mediobasal and paraventricular hypothalamus, which are predicted by our method. Among these are thyrotropin-releasing hormone (TRH)+ Arc (TRHArc) neurons, inhibitory neurons that express the Glp1r gene and are activated by the GLP-1RA liraglutide. Activating TRHArc neurons inhibits AgRP neurons and feeding, probably in an AgRP neuron-dependent manner. Silencing TRHArc neurons causes overeating and weight gain and attenuates liraglutide’s effect on body weight. Our results demonstrate a widely applicable method for molecular connectomics, comprehensively identify local inputs to AgRP neurons and reveal a circuit through which GLP-1RAs suppress appetite. Combining rabies-based connectomics with single-nucleus transcriptomics, the authors identify a neural circuit through which GLP-1 receptor agonists suppress appetite in mice.

分子连接组学揭示了胰高血糖素样肽1对饱腹感敏感的神经回路
利拉鲁肽和其他胰高血糖素样肽1受体激动剂(GLP-1RAs)是有效的减肥药,但它们如何抑制食欲仍不清楚。一种可能的机制是通过激活抑制弓形下丘脑(Arc)中促进饥饿的agouti相关肽(AgRP)神经元的神经元。为了识别这些事件,我们开发了一种结合基于狂犬病的连接组学和单核转录组学的方法。在这里,我们确定了小鼠中基底和室旁下丘脑中至少21种AgRP神经元的传入亚型,并通过我们的方法进行了预测。其中包括促甲状腺激素释放激素(TRH)+ Arc (TRHArc)神经元,表达Glp1r基因并被GLP-1RA利拉鲁肽激活的抑制性神经元。激活TRHArc神经元抑制AgRP神经元和摄食,可能以AgRP神经元依赖的方式。沉默TRHArc神经元会导致暴饮暴食和体重增加,并减弱利拉鲁肽对体重的影响。我们的研究结果展示了一种广泛适用的分子连接组学方法,全面识别AgRP神经元的局部输入,并揭示了GLP-1RAs抑制食欲的电路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


