Elemene Hydrogel Modulates the Tumor Immune Microenvironment for Enhanced Treatment of Postoperative Cancer Recurrence and Metastases
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
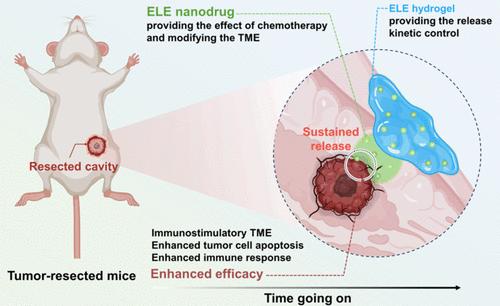
As a representative active ingredient of traditional Chinese medicine (TCM) and a clinically approved anticancer drug, elemene (ELE) exhibits exciting potential in the antitumor field; however, appropriate drug formulations still need to be explored for specific diseases such as postoperative cancer recurrence and metastasis. Herein, we report an ELE hydrogel with controlled drug release kinetics that can allow ELE to maintain effective concentrations at local lesion sites for extended periods to enhance the bioavailability of ELE. Concretely, dopamine-conjugated hyaluronic acid is synthesized and utilized to prepare ELE nanodrug-embedded hydrogels. In a model of postoperative breast cancer recurrence and metastasis, the ELE hydrogel demonstrates a 96% inhibition rate of recurrence; in contrast, the free ELE nanodrug shows only a 65.5% inhibition rate of recurrence. Importantly, the ELE hydrogel markedly stimulates a potent antitumor immune response in the microenvironment of cancer lesions, increasing antitumor immune cells such as CD8+ T cells, CD4+ T cells, and M1-type macrophages, as well as elevating antitumor cytokines including TNF-α, IFN-γ, and IL-6. Overall, this study not only advances the field of TCM but also highlights the transformative impact of controlled-release hydrogels in improving antitumor therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


