Surface Structure Dependent Activation of Hydrogen over Metal Oxides during Syngas Conversion
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
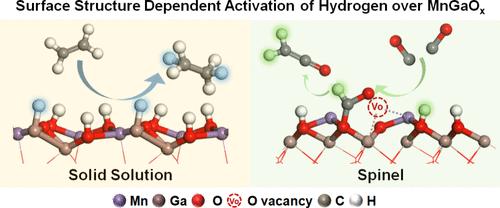
Despite the extensive studies on the adsorption and activation of hydrogen over metal oxides, it remains a challenge to investigate the structure-dependent activation of hydrogen and its selectivity mechanism in hydrogenation reactions. Herein we take spinel and solid solution MnGaOx with a similar bulk chemical composition and study the hydrogen activation mechanism and reactivity in syngas conversion. The results show that MnGaOx-Solid Solution (MnGaOx-SS) is a typical Mn-doped hexagonal close-packed (HCP) Ga2O3 with a Ga-rich surface. Upon exposure to hydrogen, Ga–H and O–H species are simultaneously generated. Ga–H species are highly active but unselective in CO activation, forming CHxO, and ethylene hydrogenation, forming ethane. In contrast, MnGaOx-Spinel is a face-centered-cubic (FCC) spinel phase featuring a Mn-rich surface, thus effectively suppressing the formation of Ga–H species. Interestingly, only part of the O–H species are active for CO activation while the O–H species are inert for olefin hydrogenation over MnGaOx-Spinel. Therefore, MnGaOx-Spinel exhibits a higher activity and higher light-olefin selectivity than MnGaOx-SS in combination with SAPO-18 during syngas conversion. These fundamental understandings are essential to guide the design and further optimization of metal oxide catalysts for selectivity control in hydrogenations.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


