Irreversible Lattice Expansion Effects in Nanoscale Indium Oxide for CO2 Hydrogenation Catalysis
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Thermal energy has been considered the exclusive driving force in thermochemical catalysis, yet associated lattice expansion effects have been overlooked. To shed new light on this issue, variable temperature in situ high-resolution (scanning) transmission electron microscopy (HR-(S)TEM) and electron energy-loss spectroscopy (EELS) were employed to provide detailed information on the structural changes of an archetype nanoscale indium oxide materials and how these effects are manifest in reverse water gas shift heterogeneous catalytic reactivity. It is found that with increasing temperature and vacuum conditions, an irreversible surface lattice expansion is traced to the formation and migration of oxygen vacancies. Together, these changes are believed to be responsible for the decreased activation energy and improved reaction rate observed for the reverse water gas shift reaction. Studies of this kind provide new insight into how thermal energy affects thermochemical heterogeneous catalysis.
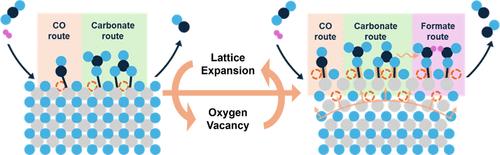
纳米氧化铟在CO2加氢催化中的不可逆晶格膨胀效应
热能一直被认为是热化学催化的唯一驱动力,但相关的晶格膨胀效应却被忽视了。为了进一步阐明这一问题,采用变温原位高分辨率(扫描)透射电子显微镜(HR-(S)TEM)和电子能量损失谱(EELS)来提供原型纳米级氧化铟材料结构变化的详细信息,以及这些影响如何在逆水气移非均相催化反应中表现出来。研究发现,随着温度和真空条件的升高,不可逆的表面晶格膨胀可追溯到氧空位的形成和迁移。总的来说,这些变化被认为是导致逆向水气转换反应活化能降低和反应速率提高的原因。这类研究为热能如何影响热化学非均相催化提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


