Impacts of informant replacement in two industry-sponsored Alzheimer's disease clinical trials
Abstract
INTRODUCTION
In Alzheimer's disease (AD) clinical trials, participants must enroll with a study partner informant who completes validated study instruments. We hypothesized that mid-trial informant replacement impacts study data in industry-sponsored trials.
METHODS
We conducted a retrospective analysis of two industry-sponsored AD clinical trials testing semagacestat in mild-to-moderate AD dementia. We assessed the relationships between informant replacement and Alzheimer's Disease Cooperative Study Activities of Daily Living (ADCS-ADL) scores. Using generalized estimating equations, we assessed bias and variability using mean (bias) and mean absolute (variance) change in ADCS-ADL between successive visits as outcomes. Both models adjusted for a priori–specified potential confounding variables including participant sex, age, informant type, trial, time, previous ADCS-ADL score, and region. To analyze the impact on end-of-study change-from-baseline results, we used an analysis of covariance model to estimate the association between replacement and end-of-study change-from-baseline in ADCS-ADL, in which we adjusted for participant sex, age, informant type, trial, baseline measurement, and region. We conducted an F-test to compare the variances of this change.
RESULTS
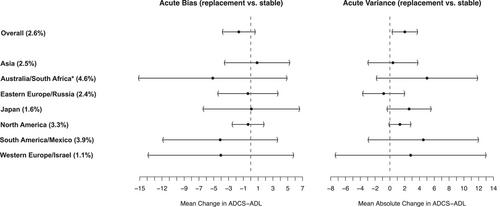
Among N = 2637 randomized participants, 69 participants (2.6%) experienced 78 occurrences of replacement. For visits standardized to be 3 months apart, the difference in mean between-visit change in ADCS-ADL was approximately −1.61 points (95% confidence interval [CI]: −3.79, 0.57; P = 0.147), comparing participants who experienced replacement to similar participants who had stable informants. The difference in the mean between-visit absolute change was approximately 2.02 points (95% CI: 0.34, 3.70; P = 0.019). We did not estimate a statistically significant difference in end-of-study change-from-baseline (Est. = −0.70 points; 95% CI: −5.88, 4.48; P = 0.790) or a significant ratio of variances (Est. = 1.13; 95% CI: 0.67, 2.28; P = 0.600) for participants with replacement compared to those with stable informants.
DISCUSSION
Informant replacement was associated with increased between-visit variability but had limited impact on overall trial outcomes.
Highlights
- Informant replacement occurred in 2.6% of participants in these industry trials.
- Informant replacement was associated with increased variance in acute Alzheimer's Disease Cooperative Study Activities of Daily Living reporting.
- Informant replacement had a limited impact on overall change-from-baseline outcomes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


