Synthesis, spectroscopical and DFT studies on third order NLO crystal: Sodium bis(malonato)borate monohydrate (SBMBM)
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The single crystal of sodium bis(malonato)borate monohydrate (SBMBM) was grown using the Slow Evaporation Solution Technique(SEST). To analyze the crystal lattice parameters of the grown SBMBM crystal, Single Crystal X-Ray Diffraction analysis were used. The results shows that the crystal belongs to Triclinic crystal system with space group P21/n with lattice parameters A = 7.9058 (4) Å, B = 8.2979 (5) Å, and C = 14.6473 (9) Å, where α = 90°, β = 101.565 (2)°, and γ = 90° The theoretical calculation utilizing HF/LANL2DZ and the experimental results agree rather well. The molecular optimized geometry, FT-IR, and the energy gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) were calculated using the HF/LANL2DZ basis set. The several functional groups in the SBMBM crystal are investigated by FT-IR spectrum. Studies of UV–visible NIR transmittance indicate that the crystal has a high transmittance throughout the whole visible spectrum. Using the Z-scan technique, third-order nonlinear optical (NLO) property of SBMBM crystal is analysed, and the linear and nonlinear refractive index are computed. χ3 = 4.49 × 10−6esu is the third-order nonlinear optical susceptibility.
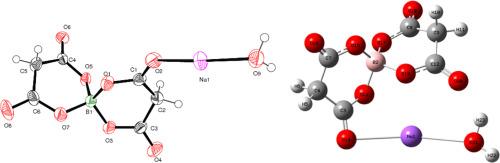
三阶硼酸钠晶体的合成、光谱和DFT研究
采用慢蒸发法制备了一水硼酸钠单晶(SBMBM)。为了分析生长的SBMBM晶体的晶格参数,采用单晶x射线衍射分析。结果表明,该晶体属于空间群为P21/n的三斜晶系,晶格参数A = 7.9058 (4) Å, B = 8.2979 (5) Å, C = 14.6473 (9) Å,其中α = 90°,β = 101.565(2)°,γ = 90°。利用HF/LANL2DZ的理论计算与实验结果吻合较好。利用HF/LANL2DZ基集计算了分子的优化几何形状、FT-IR以及最高已占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)之间的能隙。利用傅里叶红外光谱研究了SBMBM晶体中的几个官能团。紫外-可见近红外光谱透射率的研究表明,该晶体在整个可见光谱中具有较高的透射率。利用z扫描技术分析了SBMBM晶体的三阶非线性光学特性,计算了其线性折射率和非线性折射率。χ3 = 4.49 × 10−6esu为三阶非线性光学磁化率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


