Comprehensive analysis of a novel antimony-based organic-inorganic hybrid material: structural, vibrational, and hirshfeld surface investigations of (C8H14N2)[SbCl5]
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
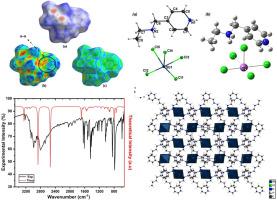
The novel organic-inorganic hybrid compound, N,N’-di(4-ethyl aminomethyl)pyridinium pentachloride antimonate (III), designated as (C8H14N2)[SbCl5], was synthesized and crystallized through a slow evaporation technique to facilitate comprehensive analysis of its crystal structure and molecular composition. Single-crystal X-ray diffraction revealed that this supramolecular compound crystallizes in the centrosymmetric space group P21/n within the orthorhombic system. The unit cell parameters were determined as follows: a = 10.8365(3) Å, b = 11.8067(3) Å, c = 12.0281(3) Å, and β = 106.920(3)°. The crystal structure was solved using direct methods and refined via the least squares technique, yielding final values of R1 = 0.0218 and wR2 = 0.0444. To further confirm the structure's stability and coherence, we investigated various interaction types and the contribution of hydrogen bonding to the overall molecular interactions through Hirshfeld surface analysis. This analysis, complemented by 2D fingerprint plots, highlighted the predominance of Cl⋯H/H⋯Cl interactions, which accounted for 59.1 % of the total interactions. Additionally, the study of crystal voids indicated that 7.1 % of the unit cell volume is composed of void space, contributing to the mechanical strength of the compound. Furthermore, FTIR spectroscopy and Raman scattering were utilized to identify the functional and molecular groups within the crystal by analyzing their vibrational modes.
一种新型锑基有机-无机杂化材料的综合分析:(C8H14N2)[SbCl5]的结构、振动和hirshfeld表面研究
合成了新型有机-无机杂化化合物N,N′-二(4-乙基氨基甲基)五氯化吡啶锑酸盐(III),命名为(C8H14N2)[SbCl5],并通过慢蒸发技术进行了结晶,便于对其晶体结构和分子组成进行综合分析。单晶x射线衍射结果表明,该超分子化合物在正交体系的中心对称空间群P21/n中结晶。测定的胞元参数为:a = 10.8365(3) Å, b = 11.8067(3) Å, c = 12.0281(3) Å, β = 106.920(3)°。采用直接法求解晶体结构,并通过最小二乘技术进行细化,得到最终值R1 = 0.0218, wR2 = 0.0444。为了进一步确认结构的稳定性和相干性,我们通过Hirshfeld表面分析研究了各种相互作用类型以及氢键对整体分子相互作用的贡献。该分析,辅以二维指纹图谱,突出了Cl⋯H/H⋯Cl相互作用的优势,占总相互作用的59.1%。此外,晶体空洞的研究表明,7.1%的单位晶胞体积是由空洞空间组成的,这有助于化合物的机械强度。此外,利用FTIR光谱和拉曼散射技术,通过分析晶体的振动模式,鉴定了晶体内部的官能团和分子基团。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


