Efficient one-pot strategy for fluorescent conjugated polymers derived from 8-amino-1-naphthalene-3,6-disulfonic acid: Synthesis, thermal and optical properties
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Schiff base polymers, also known as poly(imines) or poly(azomethine)s, constitute a subset of conjugated polymers. The Schiff base compound was synthesized via the condensation reaction between 8-amino-1-naphthalene-3,6-disulfonic acid and 4-hydroxybenzaldehyde. Subsequently, both 8-amino-1-naphthalene-3,6-disulfonic acid and its Schiff base derivative were polymerized into a poly(naphthol) (PANAPDSA) and Schiff base polymer (PANAPDSASB) under alkaline condition using H2O2 (35 % aqueous solution) as oxidant via oxidative polycondensation (OP). The chemical structures of the synthesized compounds were approved using NMR, FT-IR, UV–Vis, element and LC-MS/MS spectroscopic techniques. The synthesized polymers exhibited lower optical and electrochemical band gaps compared to their respective monomers, suggesting their potential utility as semiconductor materials. The poly(naphthol) derivative exhibited high fluorescent emission intensity of 1000 a.u. when excited at 300 nm with a photoluminescence (PL) emission quantum yield of 13.6 % at 392 nm of emission wavelength in N,N-dimethylformamide (DMF) solution. Weight average molecular weight (Mw) values of PANAPDSA and PANAPDSASB ranged from 9500 Da to 11200 Da, with PDI values between 1.12 and 1.13. The synthesis of conjugated polymers could hold significant importance in technological advancements.
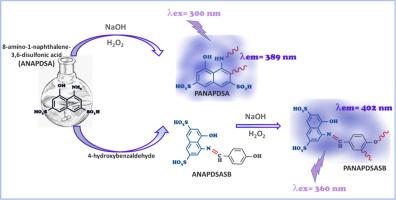
由8-氨基-1-萘-3,6-二磺酸衍生的荧光共轭聚合物的高效一锅策略:合成、热学和光学性质
希夫碱聚合物,也称为聚亚胺或聚亚甲基,是共轭聚合物的一个子集。采用8-氨基-1-萘-3,6-二磺酸与4-羟基苯甲醛缩合反应合成了希夫碱化合物。随后,以H2O2(35%水溶液)为氧化剂,在碱性条件下通过氧化缩聚(OP),将8-氨基-1-萘-3,6-二磺酸及其希夫碱衍生物聚合成聚萘酚(PANAPDSA)和希夫碱聚合物(PANAPDSASB)。采用NMR、FT-IR、UV-Vis、元素谱和LC-MS/MS等技术对合成化合物的化学结构进行了鉴定。与各自的单体相比,合成的聚合物具有更小的光学和电化学带隙,表明它们作为半导体材料的潜在用途。在N,N-二甲基甲酰胺(DMF)溶液中,当激发波长为300 nm时,聚萘酚衍生物的荧光发射强度高达1000 a.u.,在发射波长为392 nm时,发光量子产率为13.6%。PANAPDSA和PANAPDSASB的质量平均分子量(Mw)在9500 ~ 11200 Da之间,PDI在1.12 ~ 1.13之间。共轭聚合物的合成在技术进步中具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


