Synthesis and structure of trans-HfCl4(OEt2)2 and cis-ReCl4(OEt2)2, and computational studies of Group 4 to Group 7 MCl4(OEt2)2 isomer preferences (M = Zr, Hf, Nb, Ta, Mo, W, Re)
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The bis(diethyl ether) adducts of early transition metal chlorides, MCl4(OEt2)2, serve as excellent precursors for complex inorganic and organometallic compounds due to the lability of the coordinated ethers. Previously reported MCl4(OEt2)2 (M = Zr, Nb, Ta, Mo, W) complexes have crystallized with the ethers in a trans conformation, even though computational studies have predicted that compounds of the type MX4L2 should form cis isomers. Herein, we report the crystal structure of trans-HfCl4(OEt2)2 and the synthesis and structure of cis-ReCl4(OEt2)2. The report of the crystal structure of the Hf analog completes the Groups 4–6 2nd and 3rd row series and provides structural context regarding the trans preference and observations in M-Cl and M-O bond distances that are corroborated by Shannon’s ionic radii of the M(IV) cations. The isolation of the cis-Re analog provides the first structural example of a Group 7 MCl4(OEt2)2 complex, as well as the first cis complex in the presented series. Computational studies were conducted to examine the cis/trans preferences across the entire series in the context of ionic radii, ligand hardness, and steric influence.
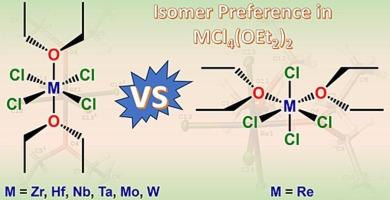
反式hfcl4 (OEt2)2和顺式cl4 (OEt2)2的合成、结构及4 ~ 7族MCl4(OEt2)2异构体偏好(M = Zr, Hf, Nb, Ta, Mo, W, Re)的计算研究
早期过渡金属氯化物的双(乙醚)加合物MCl4(OEt2)2由于配位醚的稳定性,可以作为复杂无机和有机金属化合物的优良前体。先前报道的MCl4(OEt2)2 (M = Zr, Nb, Ta, Mo, W)配合物与醚以反式构象结晶,尽管计算研究预测MX4L2类型的化合物应该形成顺式异构体。本文报道了反式hfcl4 (OEt2)2的晶体结构和顺式cl4 (OEt2)2的合成及其结构。Hf类似物的晶体结构报告完成了第4-6组第2和第3行系列,并提供了有关反式偏好的结构背景,以及M(IV)阳离子香农离子半径证实的M- cl和M- o键距离的观察结果。顺式- re类似物的分离提供了第一个7族MCl4(OEt2)2配合物的结构示例,以及本系列中的第一个顺式配合物。在离子半径、配体硬度和空间影响的背景下,进行了计算研究,以检查整个系列的顺/反式偏好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


