Exploring the enhanced bioactivity and reactivity of novel Fe(III) and Co(II) complexes incorporating sulfadiazine tetradentate ligand: Structural, DFT, molecular docking, and biological applications
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
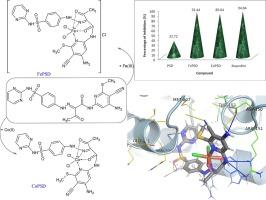
This current work presented the synthesis, characterization and bio-evaluation of new Fe(III) (FePSD), and Co(II) (CoPSD) complexes based on N-[4-amino-5-cyano-6-(methylthio)pyridin-2-yl]-3-oxobutanamide in pyridine afford N-(4-amino-5-cyano-6-(methylthio) pyridin-2-yl)-3-oxo-2-([4-(pyrimidin-2-ylsulfamoyl) phenyl]hydrazono) butanamide (PSD). Interestingly, the FePSD and CoPSD complexes exhibit notable stability and solubility in organic solvents, with molar conductivity values of 79.35 Ω−1 cm2 mol−1 for FePSD and 8.89 Ω−1 cm2 mol−1 for CoPSD, indicating a 1:1 electrolytic nature for FePSD and non-electrolytic behavior for CoPSD. FTIR analysis confirmed metal–ligand interactions, as shifts in key bands were observed, while UV–Vis spectra and magnetic moment measurements supported octahedral geometries for both complexes. DFT calculations provided insights into the electronic structure, revealing that CoPSD has higher reactivity and electrophilicity, while FePSD showed moderate reactivity. Thermal and mass spectral analyses further characterized their stoichiometry and stability. The antibacterial and antifungal activities were evaluated against a range of Gram-positive and Gram-negative bacteria and fungal strains. The FePSD and CoPSD complexes demonstrated significantly enhanced antimicrobial efficacy compared to the parent ligand, as evidenced by larger inhibition zones, higher activity indices, and lower minimum inhibition concentrations (MIC). CoPSD exhibited superior antibacterial and antifungal activity compared to FePSD, nearing the effectiveness of standard antibiotics and antifungal drugs. Additionally, the anti-inflammatory potential of these compounds was assessed using the egg albumin denaturation method. Both FePSD and CoPSD showed remarkable inhibition of protein denaturation, surpassing the parent ligand and even outperforming the standard drug Ibuprofen at higher concentrations. The enhancement in biological activity is attributed to the metal chelation effect, which improves cell membrane permeability and reactive oxygen species (ROS) generation. Molecular docking studies further elucidated the binding interactions of PSD, FePSD, and CoPSD with target proteins, revealing stronger hydrophobic interactions and hydrogen bonding in the metal complexes, correlating with their superior biological activity. These findings highlight the potential of FePSD and CoPSD as promising antimicrobial and anti-inflammatory agents.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


