USP13 inhibition exacerbates mitochondrial dysfunction and acute kidney injury by acting on MCL-1
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-11-27
DOI:10.1016/j.bbadis.2024.167599
引用次数: 0
Abstract
Acute kidney injury (AKI) is a globally recognized public health issue that lacks satisfactory therapeutic strategies. Deubiquitinase ubiquitin-specific protease 13 (USP13) regulates various pathophysiological processes via the deubiquitination of multiple substrates. However, its role in AKI remains unclear. To illustrate the role and underlying mechanism of USP13 in AKI, we subjected Usp13 knockdown mice, and mice treated with the USP13 inhibitor spautin-1, and mice with USP13 overexpression plasmids to cisplatin challenge. Renal tubular epithelial cell injury and mitochondrial disturbances were determined in vitro. Immunoprecipitation and deubiquitylation assays were performed to verify the interactions between USP13 and myeloid cell leukemia (MCL-1). We observed a significant decrease of USP13 expression in cisplatin-challenged AKI mice and renal tubular epithelial cells. Overexpression of USP13 alleviated kidney injury, whereas knockdown or inhibition of USP13 further exacerbated AKI. Mechanistically, USP13 downregulation resulted in increased degradation of MCL-1 which is a key regulator of cell survival and mitochondrial function, and the resultant MCL-1 reduction disrupted mitochondrial homeostasis and aggravated renal tubular epithelial cell injury and death, contributing to AKI progression. In conclusion, our findings demonstrated that inhibition of USP13 could exacerbate mitochondrial dysfunction and AKI through its effects on MCL-1, and USP13 may serve as a target for AKI prevention and treatment.
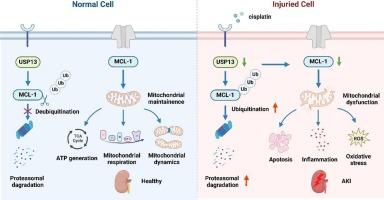
USP13抑制通过作用于MCL-1加重线粒体功能障碍和急性肾损伤。
急性肾损伤(AKI)是全球公认的公共卫生问题,缺乏令人满意的治疗策略。去泛素酶泛素特异性蛋白酶13 (USP13)通过多种底物的去泛素化调节各种病理生理过程。然而,它在AKI中的作用仍不清楚。为了阐明USP13在AKI中的作用和潜在机制,我们将USP13敲低小鼠、USP13抑制剂spautin-1治疗的小鼠以及USP13过表达质粒的小鼠置于顺铂刺激下。体外观察肾小管上皮细胞损伤和线粒体紊乱。通过免疫沉淀和去泛素化实验验证USP13与髓细胞白血病(MCL-1)之间的相互作用。我们观察到USP13在顺铂刺激的AKI小鼠和肾小管上皮细胞中的表达显著降低。USP13过表达可减轻肾损伤,而USP13敲低或抑制可进一步加重AKI。在机制上,USP13下调导致MCL-1降解增加,而MCL-1是细胞存活和线粒体功能的关键调节因子,由此导致的MCL-1减少破坏了线粒体稳态,加重了肾小管上皮细胞的损伤和死亡,导致AKI进展。综上所述,我们的研究结果表明,抑制USP13可通过其对MCL-1的影响加重线粒体功能障碍和AKI, USP13可能作为AKI预防和治疗的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


