Jingfang Granules alleviates the lipid peroxidation induced ferroptosis in rheumatoid arthritis rats by regulating gut microbiota and metabolism of short chain fatty acids
IF 4.8
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Rheumatoid arthritis (RA) is an autoimmune disease characterized by synovial inflammation, bone and cartilage damage, musculoskeletal pain, swelling, and stiffness. Inflammation is one of the key factors that induce RA. Jingfang Granule (JFG) is a traditional Chinese medicine (TCM) with significant anti-inflammatory effects. Clinical studies have confirmed that JFG can be used to treat RA, but the mechanism is still vague.
Purpose
This study was designed to evaluate the protective function and the mechanism of JFG on rats with RA.
Study design and methods
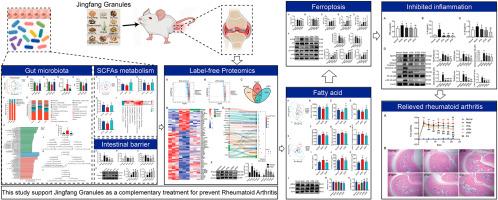
Complete Freud's Adjuvant (CFA) was used to establish a rat RA model, and JFG or Diclofenac Sodium (Dic) was orally administered. Foot swelling and hematoxylin eosin (H&E) staining were used to test the therapeutic effect of JFG on RA treatment, while ELISA kits were used to detect serum cytokines. Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), catalase (CAT), and reactive oxygen species (ROS) were used to evaluate oxidative stress levels. The integration of label-free proteomics, fecal short chain fatty acid (SCFA) targeted metabolomics, peripheral blood SCFA, medium and long chain fatty acid targeted metabolomics, and 16S rDNA sequencing of gut microbiota were used to screen the mechanism. Western blot technology was used to validate the results of multiple omics studies. Serum D-Lactic acid, lipopolysaccharide specific IgA antibody (LPS IgA), diamine oxidase (DAO), and colon Claudin 5 and ZO-1 were used to evaluate the intestinal barrier.
Results
The results confirmed that JFG effectively protected rats from RA injury, which was confirmed by improved foot swelling and synovial pathology. At the same time, JFG reduced the levels of TNF-α, IL-1β, and IL-6 in serum by inhibiting the NLRP3 inflammasome signaling pathway and TLR4/NF-κB signaling pathway in synovial tissue. Multiple omics studies indicated that JFG increased the abundance of gut microbiota and regulated the number of gut bacteria, thereby increased the levels of Acetic acid, Propionic acid, and Butyric acid in the gut and serum of RA rats, which activated AMPK to regulate fatty acid metabolism and fatty acid biosynthesis, thereby inhibited lipid oxidative stress induced ferroptosis to improve tissue damage caused by RA. Meanwhile, JFG improved the intestinal barrier by upregulating the expresses of Claudin 5 and ZO-1, which was confirmed by low concentrations of D-Lactic acid, LPS-SIgA and DAO in serum.
Conclusions
This study confirmed that JFG improved the disturbance of fatty acid metabolism by modulating gut microbiota and the production of fecal SCFAs to activate AMPK, and then inhibited ferroptosis caused by lipid oxidative stress in synovium tissue and prevented AR injury. This study proposes for the first time to investigate the mechanism of JFG treatment for RA from the perspective of the "Gut-joint" axis, and provides a promising approach for the treatment of RA.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


