Activation of receptor-independent fluid-phase pinocytosis promotes foamy monocyte formation in atherosclerotic mice
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
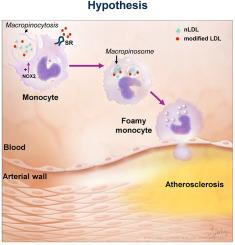
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death worldwide. Clinical and experimental data demonstrated that circulating monocytes internalize plasma lipoproteins and become lipid-laden foamy cells in hypercholesterolemic subjects. This study was designed to identify the endocytic mechanisms responsible for foamy monocyte formation, perform functional and transcriptomic analysis of foamy and non-foamy monocytes relevant to ASCVD, and characterize specific monocyte subsets isolated from the circulation of normocholesterolemic controls and hypercholesterolemic patients. We hypothesized that activation of fluid-phase macropinocytosis contributes to foamy monocyte formation in vitro and in hypercholesterolemic mice in vivo. High resolution scanning electron microscopy (SEM) and quantification of FITC/TRITC-dextran internalization demonstrated macropinocytosis stimulation in human (THP-1) and wild type murine monocytes. Stimulation of macropinocytosis induced foamy monocyte formation in the presence of unmodified, native LDL (nLDL) and oxidized LDL (ox-LDL) in vitro. Genetic blockade of macropinocytosis (LysMCre+ Nhe1f/f) inhibited foamy monocyte formation in hypercholesterolemic mice in vivo and attenuated monocyte adhesion to atherosclerotic aortas ex vivo. Mechanistic studies identified NADPH oxidase 2 (Nox2)-derived superoxide anion (O2⋅−) as an important downstream signaling molecule stimulating macropinocytosis in monocytes. qRT-PCR identified CD36 as a major scavenger receptor that increases in response to lipid loading in monocytes and deletion of CD36 (Cd36−/−) inhibited foamy monocyte formation in hypercholesterolemic mice. Bulk RNA-sequencing characterized transcriptional differences between non-foamy and foamy monocytes versus macrophages. Finally, flow cytometry analysis of CD14 and CD16 expression demonstrated a significant increase in intermediate monocytes in hypercholesterolemic patients compared to normocholesterolemic controls. These results provide novel insights into the mechanisms of foamy monocyte formation and potentially identify new therapeutic targets for the treatment of atherosclerosis.
受体不依赖的液相胞饮作用的激活促进动脉粥样硬化小鼠泡沫单核细胞的形成
动脉粥样硬化性心血管疾病(ASCVD)是世界范围内导致死亡的主要原因。临床和实验数据表明,在高胆固醇血症患者中,循环单核细胞内化血浆脂蛋白并成为富含脂质的泡沫细胞。本研究旨在确定泡沫单核细胞形成的内噬机制,对与ASCVD相关的泡沫和非泡沫单核细胞进行功能和转录组分析,并表征从正常胆固醇血症对照者和高胆固醇血症患者的循环中分离出来的特定单核细胞亚群。我们假设,在体外和体内高胆固醇血症小鼠中,激活液相巨胞增多症有助于泡沫单核细胞的形成。高分辨率扫描电镜(SEM)和FITC/ trtc -葡聚糖内化的定量显示,人(THP-1)和野生型小鼠单核细胞的巨噬细胞增多刺激。在体外未修饰的天然LDL (nLDL)和氧化LDL (ox-LDL)存在下,刺激巨噬细胞增多诱导泡沫单核细胞形成。遗传阻断巨噬细胞增多症(LysMCre+ Nhe1f/f)在体内抑制高胆固醇血症小鼠泡沫单核细胞的形成,并在体外减弱单核细胞对动脉粥样硬化主动脉的粘附。机制研究发现,NADPH氧化酶2 (Nox2)衍生的超氧阴离子(O2⋅−)是刺激单核细胞巨量红细胞增多的重要下游信号分子。qRT-PCR鉴定出CD36是一种主要的清道夫受体,在单核细胞中对脂质负荷的反应增加,在高胆固醇血症小鼠中,CD36的缺失(CD36−/−)抑制泡沫单核细胞的形成。大量rna测序表征了非泡沫和泡沫单核细胞与巨噬细胞之间的转录差异。最后,CD14和CD16表达的流式细胞术分析显示,与正常胆固醇水平的对照组相比,高胆固醇血症患者的中间单核细胞显著增加。这些结果为泡沫单核细胞形成的机制提供了新的见解,并可能为动脉粥样硬化的治疗提供新的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


