5-methyl-N'-(thiophen-2-ylmethylene)-1H-pyrazole-3-carbohydrazide as potent anticancer agent: Synthesis, spectroscopic characterization, anticancer activity and DFT studies
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
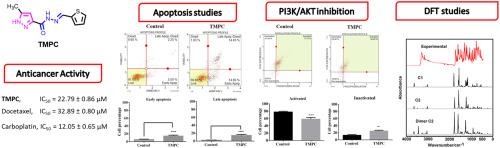
A new pyrazole-hydrazide hydrazone derivative, named 5-methyl-N'-(thiophen-2-ylmethylene)-1H-pyrazole-3-carbohydrazide (TMPC) has been synthesized and characterized by FT-IR, UV–Vis, 1H NMR, 13C NMR, and HRMS-ESI. Its cytotoxic activity was tested on various cancer and healthy cell lines using the XTT method. In addition, the advanced anticancer mechanism was investigated by flow cytometry analysis. Hybrid B3LYP/6–311++G** calculations reveal two stable C1 and C2 conformers of TMPC with energy differences of 28.96 kJ/mol in the gas phase and 8.50 kJ/mol in ethanol solution. Comparisons among the experimental NMR, UV and infrared spectra with the predicted ones support the presence of both forms in solution. The presence of dimer C2 justify the intensities and numerous bands observed in the IR spectrum in the 2000–10 cm−1 region. Probably, the repulsion between the N7 and O1 atoms in C1 explain its lower stability and the higher solvation energy (-108.20 kJ/mol) observed for this form in ethanol than C2 (-88.01 kJ/mol). NBO calculations suggest that both forms are stable in the two media while the AIM analyses evidence the higher stability of C2 than C1 due to the N9-H22···N7 interaction observed only for this conformer in the gas phase. Gap values suggest high reactivities of both forms, as compared with similar pyrazole-carbohydrazide species. Full vibrational assignments are reported for both forms of TMPC together with the main scaled force constants. The XTT results showed that TMPC exhibited a selective cytotoxic effect on the tested cancer cell lines in a dose- and time-dependent manner, and also displayed the most potent cytotoxic activity against breast cancer MCF-7 cells with an IC50 value of 22.79 µM after 48 h Flow cytometric analyses showed that TMPC induced apoptosis and suppressed PI3K/Akt signaling in MCF-7 cells.
有效抗癌剂5-甲基- n′-(噻吩-2-基亚甲基)- 1h -吡唑-3-碳酰肼:合成、光谱表征、抗癌活性和DFT研究
合成了一种新的吡唑-酰肼腙衍生物,命名为5-甲基- n′-(噻吩-2-基亚甲基)-1H-吡唑-3-碳酰肼(TMPC),并用FT-IR、UV-Vis、1H NMR、13C NMR和HRMS-ESI对其进行了表征。用XTT法测定了其对多种肿瘤和健康细胞系的细胞毒活性。此外,通过流式细胞术分析探讨了其先进的抗癌机制。混合B3LYP/ 6-311 ++G**计算得到两种稳定的TMPC C1和C2构象,它们在气相和乙醇溶液中的能量差分别为28.96 kJ/mol和8.50 kJ/mol。实验核磁共振光谱、紫外光谱和红外光谱与预测光谱的比较支持两种形式在溶液中存在。二聚体C2的存在证明了在2000-10 cm−1区域红外光谱中观察到的强度和众多波段是正确的。可能是由于C1中N7和O1原子之间的排斥力导致其在乙醇中的稳定性较低,而其在乙醇中的溶剂化能(-108.20 kJ/mol)高于C2 (-88.01 kJ/mol)。NBO计算表明,这两种形式在两种介质中都是稳定的,而AIM分析表明,由于在气相中只观察到这种构象的N9-H22···N7相互作用,C2的稳定性高于C1。间隙值表明,与类似的吡唑-碳酰肼相比,这两种形式的反应活性都很高。报告了两种形式的TMPC的全振动赋值以及主要的标度力常数。XTT实验结果显示,TMPC对乳腺癌MCF-7细胞具有剂量依赖性和时间依赖性的选择性细胞毒作用,对MCF-7细胞具有最强的细胞毒活性,48 h后IC50值为22.79µM。流式细胞术分析显示,TMPC可诱导MCF-7细胞凋亡,抑制PI3K/Akt信号通路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


