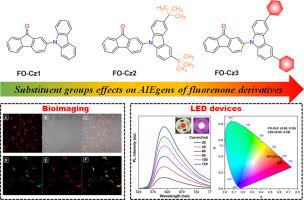
Substituent groups effects on AIEgens of fluorenone derivatives: Optical properties, bioimaging, and LED devices
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Understanding structure-property relationships is crucial for developing materials with a high fluorescence quantum yield (ΦF) and optimizing their performance. In this study, three fluorenone-carbazole-based molecules (FO-Cz1, FO-Cz2, and FO-Cz3) were synthesized, which have similar structures but varying substituent groups (carbazole, 3,6-di‑tert‑butyl‑9H-carbazole, and 3,6-diphenyl-9H-carbazole). The different substituent groups significantly changed the optical properties of the luminogens. The experimental results confirmed that FO-Cz1, FO-Cz2, and FO-Cz3 were AIEgens, and the introduction of a phenyl group gave FO-Cz3 strong αAIE (I/I0) and greater solid fluorescence quantum yields than FO-Cz1 and FO-Cz2. Analysis of photophysical properties, theoretical calculations, and X-ray crystallography revealed that the emission properties of FO-Cz1, FO-Cz2, and FO-Cz3 were influenced by the use of flexible substituent groups. The substituent groups avoided π-π stacking, reduced non-radiative transitions, and enhanced fluorescence in the aggregated state. Substituent groups on the benzene ring gave FO-Cz3 a tightly-packed structure and strong emission. It was used for cancer cell imaging and LED devices, in which FO-Cz3 exhibited high photostability and biocompatibility, making it a superior photosensitizer for bioimaging and applications in OLED devices. This research sheds light on the AIE behaviors of fluorenone complexes and offers valuable insights for the development of organic luminescent materials.
取代基对芴酮衍生物的光原的影响:光学性质、生物成像和LED器件
了解结构-性质关系对于开发具有高荧光量子产率的材料和优化其性能至关重要(ΦF)。本研究合成了三个氟酮-咔唑基分子FO-Cz1、FO-Cz2和FO-Cz3,它们具有相似的结构,但取代基不同(咔唑、3,6-二叔丁基- 9h -咔唑和3,6-二苯基- 9h -咔唑)。不同取代基显著改变了发光物质的光学性质。实验结果证实FO-Cz1、FO-Cz2和FO-Cz3均为AIEgens,并且引入一个苯基使FO-Cz3具有较强的αAIE (I/I0),固体荧光量子产率高于FO-Cz1和FO-Cz2。光物理性质分析、理论计算和x射线晶体学分析表明,FO-Cz1、FO-Cz2和FO-Cz3的发射性质受到柔性取代基使用的影响。取代基避免了π-π堆积,减少了非辐射跃迁,增强了聚集态的荧光。苯环上的取代基使FO-Cz3结构紧凑,发射性强。它被用于癌细胞成像和LED器件,其中FO-Cz3具有很高的光稳定性和生物相容性,使其成为生物成像和OLED器件应用的优越光敏剂。该研究揭示了芴酮配合物的AIE行为,为有机发光材料的开发提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


