A new chalcone derivative: Synthesis, crystal structure, Hirshfeld surface, quantum chemical investigations, Druggability and human Cathepsin D inhibitory activity
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
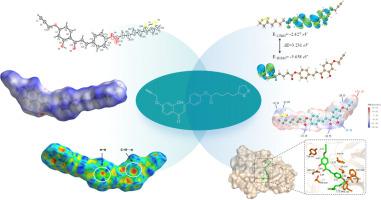
This paper presents the synthesis of a novel chalcone derivative, and its molecular structure has been determined by NMR and single-crystal X-ray diffraction analysis. The molecules are connected via O![]() H⋅⋅⋅O, C
H⋅⋅⋅O, C![]() H⋅⋅⋅O, and C
H⋅⋅⋅O, and C![]() H⋅⋅⋅S hydrogen bonds along with C
H⋅⋅⋅S hydrogen bonds along with C![]() H⋅⋅⋅π and π⋅⋅⋅π interactions. Hirshfeld surface studies have been conducted to comprehensively quantify the patterns of intermolecular interactions. DFT calculations provided insights into the nature of the molecule, including frontier molecular orbital, ADCH charge, Molecular Electrostatic Potential and Natural Bond Orbitals analysis using the optimized structure by B3LYP/6–311 G (d, p) level. The calculated HOMO-LUMO gap (3.23 eV) indicates decent softness and reactivity of the target molecule. The molecular docking studies reveal that the target compound exhibits a high binding affinity with Human Cathepsin D (CatD), with a docking score of -7.58 kcal/mol. The assessment of drug-likeness properties and ADMET (absorption, distribution, metabolism, excretion, and toxicity) profiles further support its potential as a potent CatD inhibitor candidate for medication development.
H⋅⋅⋅π and π⋅⋅⋅π interactions. Hirshfeld surface studies have been conducted to comprehensively quantify the patterns of intermolecular interactions. DFT calculations provided insights into the nature of the molecule, including frontier molecular orbital, ADCH charge, Molecular Electrostatic Potential and Natural Bond Orbitals analysis using the optimized structure by B3LYP/6–311 G (d, p) level. The calculated HOMO-LUMO gap (3.23 eV) indicates decent softness and reactivity of the target molecule. The molecular docking studies reveal that the target compound exhibits a high binding affinity with Human Cathepsin D (CatD), with a docking score of -7.58 kcal/mol. The assessment of drug-likeness properties and ADMET (absorption, distribution, metabolism, excretion, and toxicity) profiles further support its potential as a potent CatD inhibitor candidate for medication development.
一种新的查尔酮衍生物:合成、晶体结构、赫希菲尔德表面、量子化学研究、可药物性和人组织蛋白酶D抑制活性
本文合成了一种新型查尔酮衍生物,并通过核磁共振和单晶x射线衍射分析确定了其分子结构。分子通过OH、CH⋅⋅O和CH⋅⋅S氢键以及CH⋅⋅⋅π和π⋅⋅⋅⋅⋅⋅π相互作用连接。Hirshfeld表面研究已被用于全面量化分子间相互作用的模式。DFT计算提供了对分子性质的深入了解,包括分子前沿轨道、ADCH电荷、分子静电势和天然键轨道,利用优化后的结构进行B3LYP/ 6-311 G (d, p)水平分析。计算得到的HOMO-LUMO间隙为3.23 eV,表明靶分子具有良好的柔软性和反应性。分子对接研究表明,目标化合物与人组织蛋白酶D (CatD)具有较高的结合亲和力,对接评分为-7.58 kcal/mol。药物相似特性和ADMET(吸收、分布、代谢、排泄和毒性)的评估进一步支持其作为一种有效的CatD抑制剂候选药物开发的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


