Design, synthesis and characterization of novel thiazolidinone derivatives: Insights from a network pharmacology approach for breast cancer therapy
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
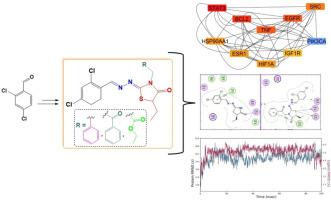
Among various types, breast cancer remains the most prevalent reason of cancer-related fatalities, underscoring its profound effect on women's health globally. In this study, we reported the synthesis and characterization of a new sequence of thiazolidinone derivatives linked to 2,4-dichlorobenzaldehyde. The compound's structures were confirmed through HRMS, while their detailed structural features were determined via IR, ¹H- and ¹³C![]() NMR spectroscopic analysis. To identify potential biological targets, network pharmacology techniques were applied to the synthesized 2,4-dichlorobenzaldehyde derivatives, which pointed to STAT3 as a primary target of interest. Additionally, the ADMET properties and molecular docking of these compounds were evaluated against the STAT3 protein. Virtual docking analyses acknowledged compound 11 as having the competent binding affinity, with a docking score of -7.87 kcal/mol, suggesting robust interactions with the key amino acid residues, Trp243 and His457. Furthermore, molecular dynamics simulation recognised the stability of compound 11, as it maintained its structural integrity within the protein binding pocket throughout the 100 ns simulation.
NMR spectroscopic analysis. To identify potential biological targets, network pharmacology techniques were applied to the synthesized 2,4-dichlorobenzaldehyde derivatives, which pointed to STAT3 as a primary target of interest. Additionally, the ADMET properties and molecular docking of these compounds were evaluated against the STAT3 protein. Virtual docking analyses acknowledged compound 11 as having the competent binding affinity, with a docking score of -7.87 kcal/mol, suggesting robust interactions with the key amino acid residues, Trp243 and His457. Furthermore, molecular dynamics simulation recognised the stability of compound 11, as it maintained its structural integrity within the protein binding pocket throughout the 100 ns simulation.
新型噻唑烷酮衍生物的设计、合成和表征:网络药理学方法对乳腺癌治疗的见解
在各种类型中,乳腺癌仍然是导致癌症相关死亡的最普遍原因,突出表明其对全球妇女健康的深远影响。在这项研究中,我们报道了一个新的序列的噻唑烷酮衍生物连接2,4-二氯苯甲醛的合成和表征。通过HRMS确定了化合物的结构,通过IR、¹H-和¹³CNMR光谱分析确定了化合物的详细结构特征。为了确定潜在的生物靶点,网络药理学技术应用于合成的2,4-二氯苯甲醛衍生物,指出STAT3是主要感兴趣的靶点。此外,对这些化合物的ADMET特性和与STAT3蛋白的分子对接进行了评估。虚拟对接分析表明,化合物11具有良好的结合亲和力,对接分数为-7.87 kcal/mol,表明与关键氨基酸残基Trp243和His457具有强大的相互作用。此外,分子动力学模拟确认了化合物11的稳定性,因为它在整个100 ns模拟中保持了其在蛋白质结合口袋内的结构完整性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


