Experimental and computational insights into antibacterial and antioxidant properties of metal complexes with isoniazid-based Schiff base ligands
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
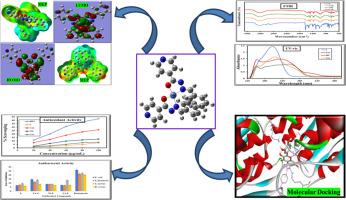
Isoniazid-based Schiff base ligands coordinate with transition metals through azomethine nitrogen and oxygen donors, enhancing electron delocalization and influencing redox properties. This structural modification impacts antibacterial and antioxidant behaviors by modulating metal-centered redox reactions, with geometry and oxidation states playing critical roles. Schiff base metal complexes derived from isoniazid and benzaldehyde were synthesized with Cu(II), Ni(II), and Co(II) ions and characterized using UV-Vis, FT-IR, NMR, and DFT analyses.
The Cu(II) complex (CuL) exhibited the highest antibacterial activity, showing a 13.83±0.44 mm inhibition zone against Staphylococcus aureus. Antioxidant activity, assessed via DPPH radical scavenging, revealed CuL as the most effective, with an IC50 of 194 µg/mL. Molecular docking studies with the MurA protein (PDB ID: 3KR6) highlighted NiL's strong binding affinity (binding energy: -10.5 kcal/mol), suggesting therapeutic potential. Frontier molecular orbital (FMO) analysis indicated lower energy gaps for CuL, NiL, and CoL, correlating with higher biological activity. These findings underscore the potential of these metal complexes as agents for combating bacterial infections and oxidative stress.
异烟肼基希夫碱配体金属配合物的抗菌和抗氧化性能的实验和计算见解
异烟肼基席夫碱配体通过氮和氧给体与过渡金属配位,增强电子离域并影响氧化还原性能。这种结构修饰通过调节以金属为中心的氧化还原反应来影响抗菌和抗氧化行为,其中几何形状和氧化态起着关键作用。以Cu(II)、Ni(II)和Co(II)离子为原料合成了异烟肼和苯甲醛的希夫贱金属配合物,并利用UV-Vis、FT-IR、NMR和DFT分析对其进行了表征。Cu(II)配合物(CuL)对金黄色葡萄球菌的抑制范围为13.83±0.44 mm,抑菌活性最高。通过清除DPPH自由基来评估抗氧化活性,显示CuL是最有效的,IC50为194µg/mL。与MurA蛋白(PDB ID: 3KR6)的分子对接研究表明,NiL具有很强的结合亲和力(结合能:-10.5 kcal/mol),具有治疗潜力。前沿分子轨道(FMO)分析表明,CuL、NiL和CoL的能隙较小,具有较高的生物活性。这些发现强调了这些金属配合物作为对抗细菌感染和氧化应激剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


