Biphenyl or naphthalene-modified fluorene fluorogenic molecules with different solid-state emission and mechanofluorochromic behaviors
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The preparation of three-color mechanofluorochromic molecules is of crucial importance in the development of mechanically responsive smart materials with multicolor switching feature. Unfortunately, the related mechano-responsive luminogenic compounds are rare. In this work, four fluorene derivatives, which contain various sites of biphenyl or naphthalene, were synthesized. Interestingly, compounds 1–4 exhibited diverse high-brightness solid-state fluorescence and observable fluorescent changes in response to mechanical force stimulus. For fluorophor 1 in the solid state, upon light grinding, an orange-yellow to yellow-green mechanofluorochromic transition with the notable blue shift of 85 nm was noticed, and the yellow-green to yellow bathochromic mechanofluorochromic response was observed after vigorous grinding. Therefore, fluorogen 1 showcased an unfrequent bidirectional tricolored mechanofluorochromism with a blue shift followed by a red shift. As for luminogens 2–4 exhibited diverse two-color reversible red-shifted mechanofluorochromic responses. Furthermore, their mechanochromic emissive mechanisms of 1–4 were investigated by X-ray powder diffraction tests.
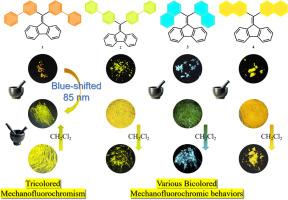
具有不同固态发射和机械荧光行为的联苯或萘修饰的芴荧光分子
三色机械荧光分子的制备对于开发具有多色开关特性的机械响应智能材料具有重要意义。不幸的是,相关的机械反应性发光化合物是罕见的。在这项工作中,合成了四种含有联苯或萘不同位点的芴衍生物。有趣的是,化合物1-4在机械力刺激下表现出不同的高亮度固态荧光和可观察到的荧光变化。对于固态的荧光团1,在光研磨时,观察到桔黄色到黄绿色的机械荧光色转变,蓝移明显为85 nm,在剧烈研磨后观察到黄绿色到黄色的机械荧光色响应。因此,氟1表现出罕见的双向三色机械荧光,先是蓝移,然后是红移。发光源2-4表现出不同的双色可逆红移机械荧光响应。通过x射线粉末衍射测试,研究了1-4的机械致色发射机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


