A theoretical comprehension of photophysical processes in Cu2+ sensing by 1,7-di(2-pyridyl)bispyrazolo[3,4-b:4′,3′-e]pyridines
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
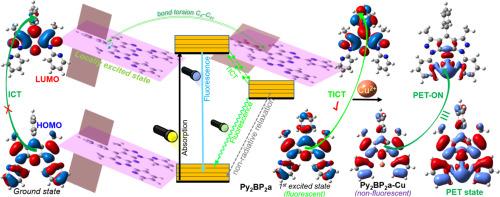
Due to its simplicity and sensitivity, metal ion sensing by fluorescent probes has a high biological and ecological impact, and several preliminary applications for Cu2+ have been found. However, the poor understanding of photophysical phenomena by which probes work has led to the growth of unhelpful literature. In this way, 4-aryl-1,7-di(pyridin-2-yl)bispyrazolo[3,4-b:4′,3′-e]pyridines Py2BP2a-c were studied as tridentate ligands in developing the probe Py2BP2a (Ar = Ph, LODCu2+ = 26 nM); thus, this previous work is completed herein by DFT/TD-DFT studies to understand the sensing process. The basal and first excited state of Py2BP2a-c (Ar: Ph, 4-An, 4-Py) and the parent l,4,7-triphenylbispyrazolo[3,4-b:3′,4′-e]pyridine Ph3BP2 were optimised. Results suggest that the probes' fluorescence is due to a twisted intramolecular charge transfer (TICT) and ICT processes around the 4-aryl and 1,7-dipyridin-2-yl groups; likewise, the fluorescence turn-off in the presence of Cu2+ by probe Py2BP2b is due to a photoinduced electron transfer (PET) process, favouring a ligand-to-metal charge transfer (LMCT). These findings enhance our understanding of the sensing process and open new possibilities for its applications in various fields.
1,7-二(2-吡啶基)双吡唑啉[3,4-b:4 ',3 ' -e]吡啶感应Cu2+光物理过程的理论理解
由于其简单和灵敏,金属离子荧光探针传感具有很高的生物和生态影响,Cu2+的几个初步应用已经被发现。然而,对探测器工作的光物理现象的理解不足导致了无益文献的增长。以4-芳基-1,7-二(吡啶-2-基)双吡唑啉[3,4-b:4 ',3 ' -e]吡啶类Py2BP2a-c为三叉戟配体,制备探针Py2BP2a (Ar = Ph, LODCu2+ = 26 nM);因此,本文通过DFT/TD-DFT研究来完成之前的工作,以了解传感过程。优化了Py2BP2a-c (Ar: Ph, 4- an,4- py)和母体1,4,7 -三苯基双吡唑[3,4-b:3 ',4 ' -e]吡啶Ph3BP2的基态和第一激发态。结果表明,探针的荧光是由于4-芳基和1,7-二吡啶-2-基周围的扭曲分子内电荷转移(TICT)和ICT过程;同样,探针Py2BP2b在Cu2+存在下的荧光关闭是由于光诱导电子转移(PET)过程,有利于配体到金属的电荷转移(LMCT)。这些发现增强了我们对传感过程的理解,并为其在各个领域的应用开辟了新的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


