Highly enhanced electro-catalytic behavior of an amide functionalized Cu(II) coordination polymer on OER at large current densities
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Extensive utilization of fossil fuels has led to their swift exhaustion, causing an escalating energy dilemma and significant environmental apprehensions. This situation has spurred advancement of sustainable energy conversion systems. Electrochemical water splitting is viewed as a capable method for generating hydrogen and oxygen intended for diverse electrochemical energy apparatus. A proficient, multifunctional electro-catalyst capable of facilitating both OERs) and HERs is of paramount importance. In this work, we report Cu-MOF electro-catalyst synthesized via the solvothermal route engaging OER activity using nickel foam (NF) in a 1 M KOH solution. Several analytical techniques were used to investigate the electro-catalysts BET, PXRD, FTIR and oxidation states. The water-splitting evaluation of the LOCOM-1 demonstrated exceptional oxygen activity concerning OER (oxygen evolution reaction), showcasing to extent a current density of 10 mAcm−2 a reduced of 286 mV over potential. Additionally, it exhibited a diminished onset potential of 1.37 V attributable towards a decreased Tafel slope of 44.50 mVdec−1, and a proton couple electron transfer (PCET) channel that is stable over an extended period of time (1000 cycles of cyclic voltammetry and 40 h of chronoamperometry). Therefore, this current endeavour might offer a new prospect or pathway for exploring the OER (Oxygen Evolution Reaction).
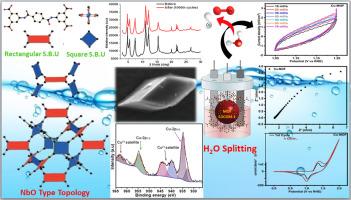
大电流密度下酰胺功能化Cu(II)配位聚合物在OER上的高增强电催化行为
化石燃料的广泛利用导致其迅速枯竭,造成日益严重的能源困境和严重的环境担忧。这种情况促使了可持续能源转换系统的发展。电化学水分解被认为是一种产生氢气和氧气的可行方法,适用于各种电化学能源装置。一个熟练的,多功能的电催化剂能够促进OERs和HERs是至关重要的。在这项工作中,我们报道了在1 M KOH溶液中使用泡沫镍(NF)通过溶剂热途径合成了具有OER活性的Cu-MOF电催化剂。采用多种分析技术对电催化剂的BET、PXRD、FTIR和氧化态进行了表征。对locom1的水分解评价表明,在OER(析氧反应)方面具有优异的氧活性,在一定程度上显示出10 mAcm−2的电流密度降低了286 mV的过电位。此外,由于Tafel斜率降低了44.50 mVdec−1,它的起始电位降低了1.37 V,并且质子对电子转移(PCET)通道在很长一段时间内保持稳定(1000次循环伏安法和40小时计时电流法)。因此,这一努力可能为探索OER(析氧反应)提供新的前景或途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


