Green synthesis of thiadiazole Schiff bases and specific recognition of Cr2O72−, SiO32− and S2−
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Sulfide (S2−), silicate (SiO32−) and dichromate (Cr2O72−) ions are prevalent contaminants in industrial effluents, and their rapid and accurate detection is of critical importance for environmental monitoring. In this study, we utilized an environmentally friendly, highly efficient, and biodegradable deep eutectic solvent (DES) to catalyze the synthesis of 2-(((5-phenyl-1,3,4-thiadiazol-2-yl)imino)methyl)phenol (Probe M). Probe M exhibits excellent selectivity for S2−, SiO32− and Cr2O72−, and the corresponding color changes can be readily detected by the naked eye. Upon addition of S2− and SiO32− to the probe solution, the solution turns a distinct orange-red, while the addition of Cr2O72− results in a pronounced darkening of the solution. The detection of S2− and SiO32− by probe M under alkaline condition is stable, and the detection of Cr2O72− under acidic condition is stable. Fluorescence titration experiments revealed that probe M forms a 1:2 stoichiometric complex with S2−, SiO32− and Cr2O72−, with binding constants of 3.84 × 104 M−1, 1.86 × 104 M−1 and 3.19 × 104 M−1, respectively. The detection limits were determined to be 1.76 × 10−4 M for S2−, 9.15 × 10−5 M for SiO32−, and 2.13 × 10−4 M for Cr2O72−. The dipstick experiment demonstrates the probe M can be used as a solid-state sensor to identify S2−, SiO32− and Cr2O72−.
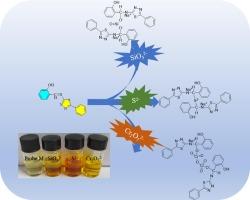
噻二唑席夫碱的绿色合成及对Cr2O72−,SiO32−和S2−的特异性识别
硫化物(S2−)、硅酸盐(SiO32−)和重铬酸盐(Cr2O72−)离子是工业废水中普遍存在的污染物,它们的快速准确检测对环境监测至关重要。在本研究中,我们利用一种环保、高效、可生物降解的深度共晶溶剂(DES)催化合成了2-((5-苯基-1,3,4-噻二唑-2-基)亚氨基)甲基)苯酚(探针M)。探针M对S2−、SiO32−和Cr2O72−具有良好的选择性,并且其颜色变化可以很容易地通过肉眼检测到。在探针溶液中加入S2−和SiO32−后,溶液变成明显的橙红色,而加入Cr2O72−则导致溶液明显变暗。M探针对S2−和SiO32−的检测在碱性条件下是稳定的,对Cr2O72−的检测在酸性条件下是稳定的。荧光滴定实验表明,探针M与S2−、SiO32−和Cr2O72−形成1:2的化学计量配合物,结合常数分别为3.84 × 104 M−1、1.86 × 104 M−1和3.19 × 104 M−1。S2−的检出限为1.76 × 10−4 M, SiO32−为9.15 × 10−5 M, Cr2O72−为2.13 × 10−4 M。量尺实验表明,探针M可以作为固态传感器来识别S2−、SiO32−和Cr2O72−。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


