Evaluating trastuzumab deruxtecan in patients with gastrooesophageal adenocarcinoma who are ctDNA and HER2 positive: DECIPHER
引用次数: 0
Abstract
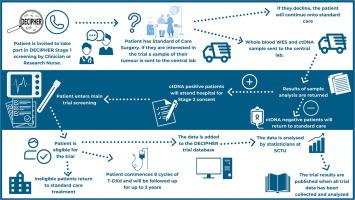
Operable gastrooesophageal adenocarcinoma (GOA) is treated with multimodality therapy which is curative in <50% of patients. Patients in the UK with operable GOA are treated with chemotherapy before and following surgery. Patients who have circulating tumour DNA (ctDNA) present after surgery have worse survival than ctDNA-negative patients. Trastuzumab deruxtecan (T-DXd), a novel human epidermal growth factor receptor 2 (HER2)-targeting antibody–drug conjugate is an effective drug in multiple tumour types and has been licensed to treat advanced HER2-positive GOA that has progressed after chemotherapy and trastuzumab and is European Society for Medical Oncology (ESMO) Guideline recommended. Evaluation of T-DXd in operable but micrometastatic GOA is an attractive option. DECIPHER is a multicentre, phase II trial testing the efficacy of T-DXd in reducing micrometastatic disease burden in HER2-positive GOA patients who are ctDNA positive after neoadjuvant chemotherapy and surgery. Patients will have their resection specimen and plasma analysed to confirm HER2 and ctDNA status post-operatively. Twenty-five ctDNA- and HER2-positive patients will be treated with 6.4 mg/kg T-DXd intravenously every 21 days for a maximum of eight cycles. Study follow-up visits will take place for a maximum of 2 years after treatment, with survival follow-up until the end of the study.
评价曲妥珠单抗在ctDNA和HER2阳性的胃食管腺癌患者中的应用:DECIPHER
可手术胃食管腺癌(GOA)的治疗采用多模式治疗,治愈率为50%。在英国,可手术的GOA患者在手术前和手术后接受化疗。术后存在循环肿瘤DNA (ctDNA)的患者比ctDNA阴性的患者生存率更差。曲妥珠单抗德鲁西替康(T-DXd)是一种新型的人表皮生长因子受体2 (HER2)靶向抗体-药物偶联物,是多种肿瘤类型的有效药物,已被许可用于治疗化疗和曲妥珠单抗后进展的晚期HER2阳性GOA,是欧洲医学肿瘤学会(ESMO)指南推荐的药物。在可手术但微转移的GOA中评估T-DXd是一个有吸引力的选择。DECIPHER是一项多中心II期试验,旨在测试T-DXd在新辅助化疗和手术后ctDNA阳性的her2阳性GOA患者中减轻微转移性疾病负担的疗效。患者将进行切除标本和血浆分析,以确认术后HER2和ctDNA状态。25例ctDNA和her2阳性患者将每21天静脉注射6.4 mg/kg T-DXd,最多8个周期。研究随访将在治疗后最多进行2年,并进行生存随访,直到研究结束。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


