Molecular dynamics insight of interaction between Artemisinin and its derivatives and the cancer cell membrane
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In the present study, a molecular dynamics simulation approach has been utilized to investigate the effectiveness of four molecules, including Artemisinin, a natural product, and its derivatives Dihydroartemisinin, Artesunate, and Artemisone, on a membrane of a cancerous cell. Performed simulations predicted that Dihydroartemisinin and Artemisone form stronger hydrogen bonds with the cancer membrane, exhibit higher mobility, and have a longer lifetime at the water-membrane interface. Artemisone molecules could penetrate the hydrophobic part of the lipid’s tail, leading to higher fluidity of the cancer membrane. These two compounds exerted the greatest effect on the properties and characteristics of the membrane model while showing stronger anti-cancer effects than the other two compounds. The simulation outcomes and predictions were found to agree with the results of experimental studies. It is noticeable that Dihydroartemisinin and Artemisone enter the cancer cell membrane from their functional group side, while Artemisinin and Artesunate enter from their peroxide ring side.
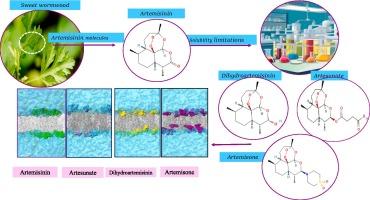
青蒿素及其衍生物与肿瘤细胞膜相互作用的分子动力学研究
在本研究中,利用分子动力学模拟方法研究了四种分子,包括天然产物青蒿素及其衍生物双氢青蒿素、青蒿琥酯和青蒿素,在癌细胞膜上的有效性。通过模拟预测,双氢青蒿素和青蒿素与癌膜形成更强的氢键,具有更高的迁移率,并且在水膜界面具有更长的寿命。青蒿素分子可以穿透脂质尾部的疏水部分,从而提高癌膜的流动性。这两种化合物对膜模型的性质和特性的影响最大,同时显示出比其他两种化合物更强的抗癌作用。模拟结果和预测结果与实验结果吻合较好。值得注意的是,双氢青蒿素和青蒿素从其官能团一侧进入癌细胞膜,而青蒿素和青蒿素从其过氧化物环一侧进入癌细胞膜。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


