N-Boron-pyrrole: A negative charge stabilizing group
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We show that the N-boron-pyrrole (B-pyrrole) group stabilizes the negatively charged compounds, despite not being an electron withdrawing group (EWG). Density functional theory (DFT) calculations revealed that this distinctive property of the B-pyrrole group arises from enhanced π-electron delocalization within the pyrrole ring. The effect of B-pyrrole group on the Brønsted and Lewis acidity of various compound classes was examined and compared with common EWGs including F, Cl, Br, CN, NO2. Substitution of pyrrole into the boracyclohexadiene derivatives enhances their acidity by about 1–6 kcal mol−1 while the acidity enhancement due to pyrrole substitution into boric acid was about 17 kcal mol−1. Interestingly, B(Pyrrole)3 exhibited a fluoride affinity comparable to BF3 (∼78 kcal mol−1) and approximately 15 kcal mol−1 higher than that of BH3.
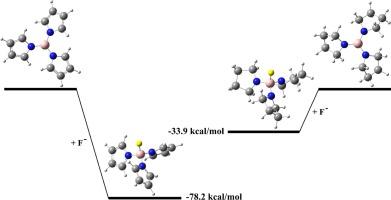
n-硼吡咯:一种负电荷稳定基团
我们发现n-硼吡咯(b -吡咯)基团稳定带负电荷的化合物,尽管不是一个吸电子基团(EWG)。密度泛函理论(DFT)计算表明,b -吡咯基团的这种特殊性质是由吡咯环内π电子离域增强引起的。考察了b -吡咯基团对各类化合物Brønsted和Lewis酸度的影响,并与F、Cl、Br、CN、NO2等常见ewg进行了比较。吡咯取代硼环己二烯衍生物使其酸度提高约1 - 6 kcal mol−1,而吡咯取代硼酸使其酸度提高约17 kcal mol−1。有趣的是,B(Pyrrole)3具有与BF3相当的氟亲和性(约78 kcal mol - 1),比BH3高约15 kcal mol - 1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


