FLASH radiotherapy combined with immunotherapy: From biological mechanisms to blockbuster therapeutics
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
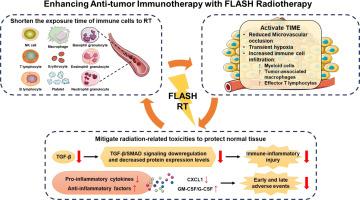
FLASH ultra-high dose rate radiotherapy (RT) can effectively exert the protective effect on normal tissue and reduce the risk of treatment-related toxicity, without compromising the killing effect on tumor tissue, resulting in a significant differential biological effect between tumor control and normal tissue damage, namely the FLASH effect. To date, the precise biological details of the FLASH effect remain uncertain. The currently mainstream mechanisms proposed by the academic community include the transient oxygen depletion hypothesis, free radical hypothesis, immune protection hypothesis, and DNA integrity hypothesis, which have attracted increasing attention in recent years. Based on these theoretical principles and numerous investigations on the FLASH effect in vivo and in vitro, the combined application of FLASH and immune checkpoint inhibitors (ICIs) has been considered synergistic and potentially practical. The primary underlying basis is that FLASH might actively preserve the number and function of circulating immune cells, thereby enhancing the efficacy of immune cell-mediated immunotherapy. Meanwhile, FLASH RT could activate the tumor immune microenvironment and transform "cold'' tumors into ''hot'' ones, consequently boosting local and systemic anti-tumor immunity and expanding the therapeutic benefits of ICIs. Moreover, FLASH might attenuate immunoinflammatory responses and minimize the incidence of radiation-related adverse events, allowing for the potentially safer and promising clinical application of combing FLASH RT with ICI therapy. Nevertheless, data on this treatment modality is currently lacking, and several barriers remain to be addressed, including the logistical bottlenecks, technical hurdles, limited availability, and unclear biological mechanisms. Further research is warranted in the future.
FLASH放疗联合免疫疗法:从生物学机制到重磅疗法
FLASH超高剂量率放疗(ultra-high dose rate radiation, RT)在不影响肿瘤组织杀伤作用的前提下,能有效发挥对正常组织的保护作用,降低治疗相关毒性的风险,从而在肿瘤控制与正常组织损伤之间产生显著的差异生物学效应,即FLASH效应。迄今为止,闪电效应的确切生物学细节仍不确定。目前学术界提出的主流机制包括瞬态耗氧假说、自由基假说、免疫保护假说和DNA完整性假说,近年来越来越受到关注。基于这些理论原理和对体内和体外FLASH效应的大量研究,FLASH和免疫检查点抑制剂(ICIs)的联合应用被认为是协同的,具有潜在的实用性。主要的潜在基础是,FLASH可能积极地保持循环免疫细胞的数量和功能,从而增强免疫细胞介导的免疫治疗的疗效。同时,FLASH RT可以激活肿瘤免疫微环境,将“冷”肿瘤转化为“热”肿瘤,从而增强局部和全身抗肿瘤免疫,扩大ICIs的治疗效果。此外,FLASH可能会减弱免疫炎症反应,并最大限度地减少辐射相关不良事件的发生率,从而允许将FLASH RT与ICI治疗相结合的潜在更安全且有前景的临床应用。然而,目前缺乏关于这种治疗方式的数据,还有几个障碍有待解决,包括后勤瓶颈、技术障碍、有限的可用性和不清楚的生物机制。未来有必要进行进一步的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


