Techno-economic analysis and process simulation of alkoxylated surfactant production in a circular carbon economy framework
IF 4.1
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
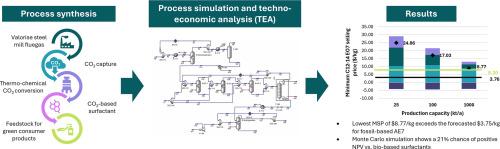
Successfully transitioning to a net-zero and circular carbon economy requires adopting innovative technologies and business models to capture CO2 and convert it into valuable chemicals and materials. Given the high economic costs and limited funding available for this transition, robust economic modelling of potential circular carbon pathways is essential to identify economically viable routes. This study introduces a novel techno-economic analysis (TEA) of producing alcohol ethoxylate (AE7), a valuable surfactant, from industrial flue gas. Traditionally, AE7 is produced by reacting fatty alcohols with ethylene oxide derived from fossil or bio-based sources. This research explores a method using CO2 captured from steel industry flue gas to produce AE7, addressing a notable gap in the literature. It evaluates a thermo-catalytic pathway involving Fischer-Tropsch (FT) synthesis with syngas generated by the reverse-water gas-shift reaction, where CO2 reacts with H2. CO2 conversion rates range around 3% across processing capacities of 25 kt/a, 100 kt/a, and 1000 kt/a. The study finds that the CO2 mass fraction concentration in the process emission is 2.47 × 10–5, compared to 0.13 in the incoming flue gas, highlighting the system's positive environmental impact. A radial basis function neural network was built to forecast the long-term average price of fossil-based and bio-based surfactants to benchmark the results against. Economic analysis reveals that the cost of green hydrogen significantly impacts the minimum selling price (MSP), making cost parity with existing fossil-based surfactants challenging. The lowest MSP of $8.77/kg remains above the long-term forecasted price of $3.75/kg for fossil-based C12–14 AE7. However, Monte Carlo simulations show a 21% probability of achieving a positive net present value (NPV) compared to leading bio-based surfactant alternatives. Sensitivity analyses identify capital costs, the price of low-carbon hydrogen (LCOH), and diesel prices as the most influential factors affecting the MSP. Continued advancements in Fischer-Tropsch catalyst technologies, reductions in green hydrogen costs and growing consumer demand for environmentally friendly products could significantly enhance the economic feasibility of this sustainable approach, paving the way for broader adoption and contributing to a circular carbon economy.
循环碳经济框架下烷氧基表面活性剂生产的技术经济分析与工艺模拟
成功过渡到净零碳和循环碳经济需要采用创新的技术和商业模式来捕获二氧化碳并将其转化为有价值的化学品和材料。鉴于这种转变的经济成本高,可用资金有限,对潜在的循环碳途径进行强有力的经济建模对于确定经济上可行的路线至关重要。介绍了一种新的技术经济分析方法,即从工业烟气中提取有价值的表面活性剂乙醇乙氧基酸酯(AE7)。传统上,AE7是由脂肪醇与来自化石或生物基来源的环氧乙烷反应产生的。本研究探索了一种利用从钢铁工业烟气中捕获的二氧化碳生产AE7的方法,解决了文献中一个显着的空白。它评估了一种热催化途径,包括用逆水气移反应产生的合成气进行费托合成(FT),其中CO2与H2反应。在25 kt/a、100 kt/a和1000 kt/a的处理能力中,二氧化碳转化率约为3%。研究发现,过程排放中的CO2质量分数浓度为2.47 × 10-5,而进入烟气中的CO2质量分数浓度为0.13,突出了系统对环境的积极影响。建立了径向基函数神经网络来预测化石基和生物基表面活性剂的长期平均价格,并以此作为基准。经济分析表明,绿色氢的成本对最低销售价格(MSP)有很大影响,这使得与现有化石基表面活性剂的成本相当具有挑战性。最低MSP为8.77美元/公斤,仍高于化石燃料C12-14 AE7的长期预测价格3.75美元/公斤。然而,蒙特卡罗模拟显示,与领先的生物基表面活性剂替代品相比,实现正净现值(NPV)的可能性为21%。敏感性分析发现,资本成本、低碳氢(LCOH)价格和柴油价格是影响MSP的最重要因素。费托催化剂技术的持续进步、绿色氢成本的降低以及消费者对环保产品需求的不断增长,可以显著提高这种可持续方法的经济可行性,为更广泛的采用铺平道路,并为循环碳经济做出贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


