The impact of Al/Cr ratio on the oxidation kinetics of Y-doped AlCoCrFeNi high-entropy alloys at 1100 °C
IF 4.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Y/Hf-doped AlCoCrFeNi high-entropy alloys stand out for their potential application in high temperature coatings. Thereinto, both Cr and Al are crucial for improving oxidation properties. However, simultaneously increasing the content of Al and Cr is not advisable, since it can significantly reduce the ductility/toughness of the coating. In this research, we proposed an equivalent replacement method of Al and Cr, namely, tuning Al/Cr ratio (ACR), to enhance the elevated-temperature oxidation resistance of AlCoCrFeNi alloys. This strategy was verified by the 1000 h/1100 °C oxidation tests of three Y-doped AlCoCrFeNi alloys with different ACRs of 0.78, 0.58 and 0.41. The test results indicated an elusive transformation of oxidation rate occurred on these alloys, that the alloy with lowest ACR exhibited an initially higher oxidation rate but a lower oxidation rate over an extended period, in comparison to those higher ACR alloys. The underlying oxidation mechanisms were uncovered using microscopic techniques and thermodynamics calculations. The initial higher oxidation rate was ascribed to the rapid growth of spinel oxides, while the extended slower oxidation process was attributed to the resulting Al2O3 scale with larger grain sizes. Thermodynamic assessment revealed that larger Al2O3 grains corresponding to fewer grain boundaries decreased the diffusion coefficient of oxygen in Al2O3 scale. Our research is of both theoretical and industrial importance for clarifying the high temperature oxidation mechanism of Y-doped AlCoCrFeNi alloys and enhancing the oxidation resistance in multicomponent alloy systems.
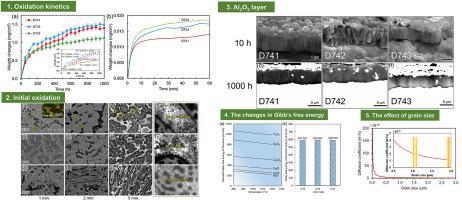
Al/Cr比对y掺杂AlCoCrFeNi高熵合金1100℃氧化动力学的影响
Y/ hf掺杂的AlCoCrFeNi高熵合金在高温涂层中具有潜在的应用前景。其中,Cr和Al都是改善氧化性能的关键。然而,同时增加Al和Cr的含量是不可取的,因为它会显著降低涂层的延展性/韧性。在本研究中,我们提出了Al和Cr的等效替代方法,即调整Al/Cr比(ACR),以提高AlCoCrFeNi合金的高温抗氧化性。通过acr分别为0.78、0.58和0.41的三种掺y AlCoCrFeNi合金的1000 h/1100℃氧化试验验证了该策略。试验结果表明,这些合金的氧化速率发生了难以捉摸的转变,与ACR较高的合金相比,ACR最低的合金最初的氧化速率较高,但在较长一段时间内氧化速率较低。利用微观技术和热力学计算揭示了潜在的氧化机制。最初较高的氧化速率归因于尖晶石氧化物的快速生长,而延长的较慢的氧化过程归因于由此产生的晶粒尺寸较大的Al2O3垢。热力学评价表明,Al2O3晶粒越大,晶界越小,氧在Al2O3垢中的扩散系数降低。我们的研究对于阐明y掺杂AlCoCrFeNi合金的高温氧化机理,提高多组分合金体系的抗氧化性具有重要的理论和工业意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Intermetallics
工程技术-材料科学:综合
CiteScore
7.80
自引率
9.10%
发文量
291
审稿时长
37 days
期刊介绍:
This journal is a platform for publishing innovative research and overviews for advancing our understanding of the structure, property, and functionality of complex metallic alloys, including intermetallics, metallic glasses, and high entropy alloys.
The journal reports the science and engineering of metallic materials in the following aspects:
Theories and experiments which address the relationship between property and structure in all length scales.
Physical modeling and numerical simulations which provide a comprehensive understanding of experimental observations.
Stimulated methodologies to characterize the structure and chemistry of materials that correlate the properties.
Technological applications resulting from the understanding of property-structure relationship in materials.
Novel and cutting-edge results warranting rapid communication.
The journal also publishes special issues on selected topics and overviews by invitation only.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


