Suppression of ADP-ribosylation reversal triggers cell vulnerability to alkylating agents
IF 4.8
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
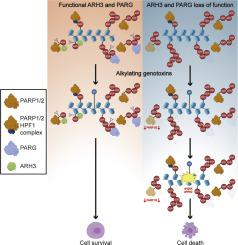
The ADP-ribosyl hydrolases PARG and ARH3 counteract PARP enzymatic activity by removing ADP-ribosylation. PARG and ARH3 activities have a synthetic lethal effect; however, the specific molecular mechanisms underlying this response remain unknown. Here, we show that the PARG and ARH3 synthetic lethality is enhanced further in the presence of DNA alkylating agents, suggesting that the inability to revert ADP-ribosylation primarily affects the repair of alkylated DNA bases. ARH3 knockout cells, treated with PARG inhibitor and alkylating genotoxins, accumulated single-stranded DNA and DNA damage, resulting in G2/M cell cycle arrest and apoptosis. Furthermore, we reveal a reduction in PARP1/PARP2 levels in ARH3-deficient cells treated with PARG inhibitor due to excessive ADP-ribosylation, which may contribute to alkylating agents’ vulnerability. Collectively, these results uncover the potential of targeting ADP-ribosyl hydrolases in combination with alkylating agents for cancer therapy and provide insights into the mechanisms underlying the synthetic lethal effect.
抑制adp核糖基化逆转触发细胞对烷基化剂的脆弱性
adp -核糖基水解酶PARG和ARH3通过去除adp -核糖基化来中和PARP酶的活性。PARG和ARH3活性具有合成致死作用;然而,这种反应的具体分子机制尚不清楚。在这里,我们发现在DNA烷基化剂的存在下,PARG和ARH3的合成致死率进一步增强,这表明无法恢复adp核糖基化主要影响烷基化DNA碱基的修复。ARH3敲除细胞经PARG抑制剂和烷基化基因毒素处理后,单链DNA和DNA损伤积累,导致G2/M细胞周期阻滞和凋亡。此外,我们发现在PARG抑制剂处理的arh3缺陷细胞中,由于adp核糖基化过度,PARP1/PARP2水平降低,这可能有助于烷基化剂的脆弱性。总的来说,这些结果揭示了靶向adp -核糖基水解酶与烷基化剂联合用于癌症治疗的潜力,并为合成致死效应的机制提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


