TPM4 overexpression drives colon epithelial cell tumorigenesis by suppressing differentiation and promoting proliferation
IF 4.8
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
Objective
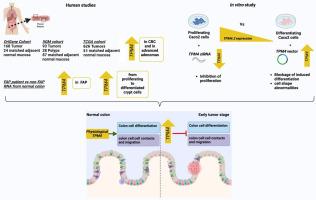
The high morbidity and mortality associated with colorectal cancer (CRC) and the recent increases in early-onset CRC obviate the need for novel methods to detect and treat this disease, particularly at early stages. We hypothesize that aberrant expression of genes involved in the crypt-luminal migration of colon epithelial cells, a process necessary for their growth arrest and maturation, may disrupt differentiation and transition cells from a normal to tumorigenic state.
Methods
We searched for contractility- and motility-related genes that are dysregulated in human CRC relative to normal colon. RNA expression of one such gene, tropomyosin 4 (TPM4), was measured by qRT-PCR and RNA-seq in human colorectal tissues at various stages of tumorigenesis: CRC, adenoma, and at-risk (grossly normal mucosa from a patient with Familial Adenomatous Polyposis, or FAP), relative to controls. Effects of aberrant TPM4 expression on colon epithelial cell proliferation and maturation were determined by overexpression using stable transfection in spontaneously differentiating Caco2 cells or silencing using siRNA in proliferating cells.
Results
TPM4 is overexpressed at various stages of tumorigenesis, including CRC, adenoma, and grossly normal FAP colon tissue, as well as in proliferating versus differentiating Caco2 cells. TPM4.2 overexpression in differentiating Caco2 cells markedly inhibits certain aspects of maturation, notably sucrase isomaltase and glutathione-S-transferase alpha1 expression, and causes morphological and cell junction abnormalities. Conversely, siRNA-mediated suppression of TPM4.2 inhibits Caco2 proliferation.
Conclusions
TPM4 overexpression attenuates colon epithelial cell differentiation and promotes proliferation. Therefore, TPM4 expression may be a biomarker to enhance strategies for CRC diagnosis and treatment.
TPM4过表达通过抑制分化和促进增殖驱动结肠上皮细胞的肿瘤发生
目的结直肠癌(CRC)的高发病率和死亡率以及近年来早发性CRC的增加,使得对这种疾病的检测和治疗的新方法的需求,特别是在早期阶段。我们假设,参与结肠上皮细胞隐窝-腔内迁移的基因的异常表达可能会破坏细胞从正常状态向致瘤状态的分化和转化。结肠上皮细胞隐窝-腔内迁移是其生长停滞和成熟所必需的过程。方法我们在人类结直肠癌中寻找相对于正常结肠的收缩性和运动性相关基因。其中一种基因原肌球蛋白4 (TPM4)的RNA表达,通过qRT-PCR和RNA-seq在不同肿瘤发生阶段的人类结直肠组织中相对于对照组进行了测量:CRC、腺瘤和高危(来自家族性腺瘤性息肉病(FAP)患者的大体正常粘膜)。TPM4异常表达对结肠上皮细胞增殖和成熟的影响通过在自发分化的Caco2细胞中稳定转染过表达或在增殖细胞中使用siRNA沉默来确定。结果stpm4在肿瘤发生的各个阶段都过表达,包括结直肠癌、腺瘤和大体正常的FAP结肠组织,以及增殖和分化的Caco2细胞。TPM4.2在分化cca2细胞中的过表达会显著抑制成熟过程的某些方面,特别是蔗糖酶异麦芽糖酶和谷胱甘肽- s -转移酶alpha1的表达,并导致形态和细胞连接异常。相反,sirna介导的TPM4.2抑制可抑制Caco2增殖。结论stpm4过表达可减缓结肠上皮细胞分化,促进细胞增殖。因此,TPM4的表达可能是一种生物标志物,可以增强CRC的诊断和治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


