CCR1 antagonist as a potential modulator of inflammatory, autophagic, and apoptotic markers in spinal cord injury
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
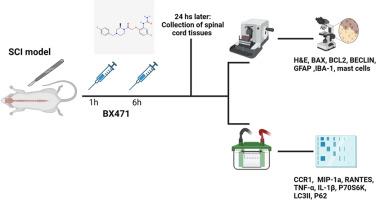
Spinal cord injury (SCI) leads to severe and lasting impairments in motor and sensory functions. The intense inflammatory response following SCI is a significant challenge, and autophagy has emerged as a key factor in the recovery process. The C-C chemokine receptor type 1 (CCR1), a G-protein coupled receptor, plays a crucial role in managing the chemokine response under stress. BX471, a selective and potent CCR1 antagonist, has been explored in various disease contexts for its therapeutic potential. In this study, we assessed the effects of BX471 in a mouse model of SCI. The treatment was administered at doses of 3 and 10 mg/kg, 1 h and 6 h after the injury occurred. Results showed that BX471 significantly improved tissue structure by positively influencing autophagy and reducing inflammation. Inflammatory markers, including CCR1 ligands RANTES, MIP-1α, TNF-α, and IL-1β, were measured using Western blot analysis. Additionally, histological evaluations revealed that BX471 effectively decreased infiltration and reduced astrocyte and microglial activation, supporting the idea that enhancing autophagy through CCR1 inhibition could promote neuronal survival. The highest efficacy was observed at the 10 mg/kg dose, leading to optimal out-comes across the assessments. These findings suggest that CCR1 blockade with BX471 may offer a promising therapeutic strategy for SCI, addressing a critical gap in the current pharmacological treatment options.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


